Empagliflozin
(Jardiance®)
Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitor
1. Product description
Empagliflozin (Jardiance) film-coated tablets, 10 mg are pale yellow, round, biconvex and bevel-edged film-coated tablets. One side is debossed with the code ‘S10’, the other side is debossed with the Boehringer Ingelheim company symbol.
Empagliflozin (Jardiance) film-coated tablets, 25 mg are pale yellow, oval, biconvex film-coated tablets. One side is debossed with the code ‘S25’, the other side is debossed with the Boehringer Ingelheim company symbol.
2. Formulation
1 film-coated tablet contains……………………………………………………………………10, 25 mg D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl] oxy]phenyl]methyl]phenyl]-, (1S) (= empagliflozin)
Excipients: Lactose monohydrate, Cellulose microcrystalline, Hydroxypropyl cellulose, Croscarmellose sodium, Silica, colloidal anhydrous, Magnesium stearate, Opadry® Yellow 02B38190.
3. Indications/Usage
Type 2 diabetes mellitus
Glycaemic control:
Empagliflozin (Jardiance) is indicated in the treatment of type 2 diabetes mellitus to improve glycaemic control in adults as:
Monotherapy
When diet and exercise alone do not provide adequate glycaemic control in patients for whom use of metformin is considered inappropriate due to intolerance.
Add-on combination therapy
In combination with other glucose–lowering medicinal products including insulin, when these, together with diet and exercise, do not provide adequate glycaemic control (see Clinical Trials).
Prevention of cardiovascular death
Empagliflozin (Jardiance) is indicated in patients with type 2 diabetes mellitus and established cardiovascular disease to reduce the risk of cardiovascular death (see Clinical Trials).
To prevent cardiovascular deaths, Empagliflozin (Jardiance) should be used in conjunction with other measures to reduce cardiovascular risk in line with the current standard of care.
Heart failure (HF)
Empagliflozin (Jardiance) is indicated in adult patients with heart failure (NYHA class II-IV) independent of left ventricular ejection fraction, with or without type 2 diabetes mellitus:
- to reduce the risk of cardiovascular death and hospitalisation for heart failure
- to slow kidney function decline
Chronic kidney disease
Empagliflozin (Jardiance) is indicated in adult patients with chronic kidney disease to reduce the risk of:
- Kidney disease progression (sustained decline in estimated glomerular filtration rate (eGFR), end-stage kidney disease or renal death) or cardiovascular death
- All-cause hospitalisation
4. Dosage and administration
Type 2 diabetes mellitus
The recommended starting dose of Empagliflozin (Jardiance) is 10 mg once daily.
In patients tolerating empagliflozin 10 mg once daily who have an eGFR ≥ 30 mL/min/1.73 m2 and requiring additional glycaemic control, the dose can be increased to 25 mg once daily.
Heart failure
The recommended dose of Empagliflozin (Jardiance) is 10 mg once daily (see clinical trial section).
Chronic kidney disease
The recommended dose of Empagliflozin (Jardiance) is 10 mg once daily (see clinical trial section).
Empagliflozin (Jardiance) can be taken with or without food.
Patients with renal impairment
Empagliflozin 10 mg can be used regardless of renal function. However, due to limited experience, it is not recommended to initiate treatment with Empagliflozin (Jardiance) in patients on dialysis.
Glycaemic efficacy of empagliflozin is dependent on renal function and likely absent in patients with severe renal impairment. If eGFR falls below 30 mL/min/1.73 m2 the recommended dose of empagliflozin is limited to 10 mg and additional glucose lowering treatment should be considered if needed (see Special warnings and precautions).
Patients with hepatic impairment
No dose adjustment is recommended for patients with hepatic impairment.
Elderly patients
No dosage adjustment is recommended based on age.
Combination therapy
When Empagliflozin (Jardiance) is used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia. (see sections Interactions and Adverse Reactions).
Missed dose
If a dose is missed, it should be taken as soon as the patient remembers. A double dose should not be taken on the same day.
Paediatric population
Safety and effectiveness of Empagliflozin (Jardiance) in children under 18 years of age have not been established.
5. Contraindications
Hypersensitivity to empagliflozin or any of the excipients.
In case of rare hereditary conditions that may be incompatible with an excipient of the product (please refer to section Special warnings and precautions), the use of the product is contraindicated.
6. Special warnings and precautions
Empagliflozin (Jardiance) should not be used in patients with type 1 diabetes.
Ketoacidosis
Cases of ketoacidosis, a serious life-threatening condition requiring urgent hospitalisation, have been reported in patients with diabetes mellitus treated with empagliflozin, including fatal cases. In a number of reported cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14 mmol/L (250 mg/dL). Although ketoacidosis is less likely to occur in patients without diabetes mellitus, cases have also been reported in these patients.
The risk of ketoacidosis must be considered in the event of non-specific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness.
Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level. If ketoacidosis is suspected, Empagliflozin (Jardiance) should be discontinued, patient should be evaluated, and prompt treatment should be instituted.
Patients who may be at higher risk of ketoacidosis while taking Empagliflozin (Jardiance) include patients on a very low carbohydrate diet (as the combination may further increase ketone body production), patients with an acute illness, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), insulin dose reduction (including insulin pump failure), alcohol abuse, severe dehydration, and patients with a history of ketoacidosis. Empagliflozin (Jardiance) should be used with caution in these patients. When reducing the insulin dose (see Dosage and Administration), caution should be taken. In patients treated with Empagliflozin (Jardiance) consider monitoring for ketoacidosis and temporarily discontinuing Empagliflozin (Jardiance) in clinical situations known to predispose to ketoacidosis (e.g. prolonged fasting due to acute illness or surgery). In these situations, consider monitoring of ketones, even if Empagliflozin (Jardiance) treatment has been interrupted.
Necrotizing fasciitis of the perineum (Fournier’s gangrene)
Cases of necrotizing fasciitis of the perineum (also known as Fournier’s gangrene), a rare, but serious and life-threatening necrotizing infection, have been reported in female and male patients with diabetes mellitus treated with SGLT2 inhibitors, including empagliflozin. Serious outcomes have included hospitalisation, multiple surgeries, and death.
Patients treated with Empagliflozin (Jardiance) who present with pain or tenderness, erythema, swelling in the genital or perineal area, fever, malaise should be evaluated for necrotizing fasciitis. If suspected, Empagliflozin (Jardiance) should be discontinued and prompt treatment should be instituted (including broad-spectrum antibiotics and surgical debridement if necessary).
Use in patients with renal impairment
Due to limited experience, it is not recommended to initiate treatment with empagliflozin in patients on dialysis.
Glycaemic efficacy of empagliflozin is dependent on renal function and likely absent in patients with an eGFR <30 mL/min/1.73 m2 (see Dosage and administration).
Monitoring of renal function
Assessment of renal function is recommended prior to Empagliflozin (Jardiance) initiation and periodically during treatment, i.e., at least yearly.
Use in patients at risk for volume depletion
Based on the mode of action of SGLT2 inhibitors, osmotic diuresis accompanying glucosuria may lead to a modest decrease in blood pressure. Therefore, caution should be exercised in patients for whom an empagliflozin-induced drop in blood pressure could pose a risk, such as patients with known cardiovascular disease, patients on anti-hypertensive therapy with a history of hypotension or patients aged 75 years and older.
In case of conditions that may lead to fluid loss (e.g. gastrointestinal illness), careful monitoring of volume status (e.g. physical examination, blood pressure measurements, laboratory tests including haematocrit) and electrolytes is recommended for patients receiving empagliflozin. Temporary interruption of treatment with Empagliflozin (Jardiance) should be considered until the fluid loss is corrected.
Complicated urinary tract infections
Cases of complicated urinary tract infections including pyelonephritis and urosepsis have been reported in patients treated with empagliflozin (see Adverse Reactions). Temporary interruption of Empagliflozin (Jardiance) should be considered in patients with complicated urinary tract infections.
Elderly patients
Patients aged 75 years and older may be at increased risk of volume depletion, therefore, Empagliflozin (Jardiance) should be prescribed with caution in these patients (see Adverse Reactions).
Lactose
Empagliflozin (Jardiance) 10 mg and 25 mg tablets contain 162.5 mg and 113 mg of lactose per maximum recommended daily dose, respectively. Patients with the rare hereditary conditions of galactose intolerance e.g. galactosaemia should not take this medicine.
Sodium
Empagliflozin (Jardiance) contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially 'sodium free'.
7. Use in specific populations
Pregnancy, lactation and fertility
Pregnancy
There are limited data from the use of Empagliflozin (Jardiance) in pregnant women. Nonclinical studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure it is recommended to avoid the use of Empagliflozin (Jardiance) during pregnancy unless clearly needed.
Lactation
No data in humans are available on excretion of empagliflozin into milk. Available nonclinical data in animals have shown excretion of empagliflozin in milk. A risk to human newborns/infants cannot be excluded. It is recommended to discontinue breast feeding during treatment with Empagliflozin (Jardiance).
Fertility
No studies on the effect on human fertility have been conducted for Empagliflozin (Jardiance). Nonclinical studies in animals do not indicate direct or indirect harmful effects with respect to fertility.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed.
8. Interactions
Pharmacodynamic interactions
Diuretics
Empagliflozin may add to the diuretic effect of thiazide and loop diuretics and may increase the risk of dehydration and hypotension.
Insulin and insulin secretagogues
Insulin and insulin secretagogues, such as sulphonylureas, may increase the risk of hypoglycaemia. Therefore, a lower dose of insulin or an insulin secretagogue may be required to reduce the risk of hypoglycaemia when used in combination with empagliflozin (see sections Dosage and Administration and Adverse Reactions).
Interference with 1,5-anhydroglucitol (1,5-AG) Assay
Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.
Pharmacokinetic interactions
Lithium
Concomitant use of SGLT2 inhibitors, including empagliflozin, with lithium may decrease blood lithium levels through increased renal lithium elimination. Therefore, serum lithium concentration should be monitored more frequently with empagliflozin initiation or following dose changes. Please refer the patient to the lithium prescribing doctor in order to monitor serum concentration of lithium.
In vitro assessment of drug interactions
Empagliflozin does not inhibit, inactivate, or induce CYP450 isoforms. In vitro data suggest that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9. Empagliflozin does not inhibit UGT1A1, UGT1A3, UGT1A8, UGT1A9, or UGT2B7. At therapeutic doses, the potential for empagliflozin to reversibly inhibit or inactivate the major CYP450 and UGT isoforms is remote. Drug-drug interactions involving the major CYP450 and UGT isoforms with empagliflozin and concomitantly administered substrates of these enzymes are therefore considered unlikely.
Empagliflozin is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), but it does not inhibit these efflux transporters at therapeutic doses. Based on in vitro studies, empagliflozin is considered unlikely to cause interactions with drugs that are P-gp substrates. Empagliflozin is a substrate of the human uptake transporters OAT3, OATP1B1, and OATP1B3, but not OAT1 and OCT2. Empagliflozin does not inhibit any of these human uptake transporters at clinically relevant plasma concentrations and, as such, drug-drug interactions with substrates of these uptake transporters are considered unlikely.
In vivo assessment of drug interactions
No clinically meaningful pharmacokinetic interactions were observed when empagliflozin was co-administered with other commonly used medicinal products. Based on results of pharmacokinetic studies no dose adjustment of Empagliflozin (Jardiance) is recommended when co-administered with commonly prescribed medicinal products.
Empagliflozin pharmacokinetics were similar with and without co-administration of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, verapamil, ramipril, simvastatin, in healthy volunteers and with or without co-administration of torasemide and hydrochlorothiazide in patients with T2DM. Increases in overall exposure (AUC) of empagliflozin were seen following co-administration with gemfibrozil (59%), rifampicin (35%), or probenecid (53%). These changes were not considered to be clinically meaningful.
Empagliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, digoxin, ramipril, simvastatin, hydrochlorothiazide, torasemide and oral contraceptives when co-administered in healthy volunteers.
9. Adverse reactions
Type 2 diabetes mellitus
A total of 15,582 patients with type 2 diabetes were treated in clinical studies to evaluate the safety of empagliflozin, of which 10,004 patients were treated with empagliflozin, either alone or in combination with metformin, a sulfonylurea, a PPARγ agonist, DPP-4 inhibitors, or insulin. This pool includes the EMPA-REG OUTCOME® study involving 7,020 patients at high cardiovascular risk (mean age 63.1 years, 9.3% patients at least 75 years old, 28.5% women) treated with Empagliflozin (Jardiance) 10 mg/day (n=2345), Empagliflozin (Jardiance) 25 mg/day (n=2342), or placebo (n=2333) up to 4.5 years. The overall safety profile of empagliflozin in this study was comparable to the previously known safety profile. In the above described trials, the frequency of AEs leading to discontinuation was similar by treatment groups for placebo, Empagliflozin (Jardiance) 10 mg and Empagliflozin (Jardiance) 25 mg.
Placebo-controlled double-blind trials of 18 to 24 weeks of exposure included 3534 patients, of which 1183 were treated with placebo, 1185 were treated with Empagliflozin (Jardiance) 10 mg and 1166 were treated with Empagliflozin (Jardiance) 25 mg.
The most frequent adverse drug reaction was hypoglycaemia, which depended on the type of background therapy used in the respective studies (see description of selected adverse reactions).
Heart failure
The EMPEROR studies included patients with heart failure and either reduced ejection fraction (N = 3726) or preserved ejection fraction (N = 5985) treated with 10 mg empagliflozin or placebo. Approximately half of the patients had type 2 diabetes mellitus.
The most frequent adverse drug reaction was volume depletion (empagliflozin 10 mg: 11.4%; placebo: 9.7%).
Chronic kidney disease
The EMPA-KIDNEY study included patients with chronic kidney disease (N = 6609) treated with 10 mg empagliflozin or placebo. About 44% of the patients had type 2 diabetes mellitus.
No new adverse reactions were identified in the EMPA-KIDNEY study.
The overall safety profile of Empagliflozin (Jardiance) was generally consistent across the studied indications.
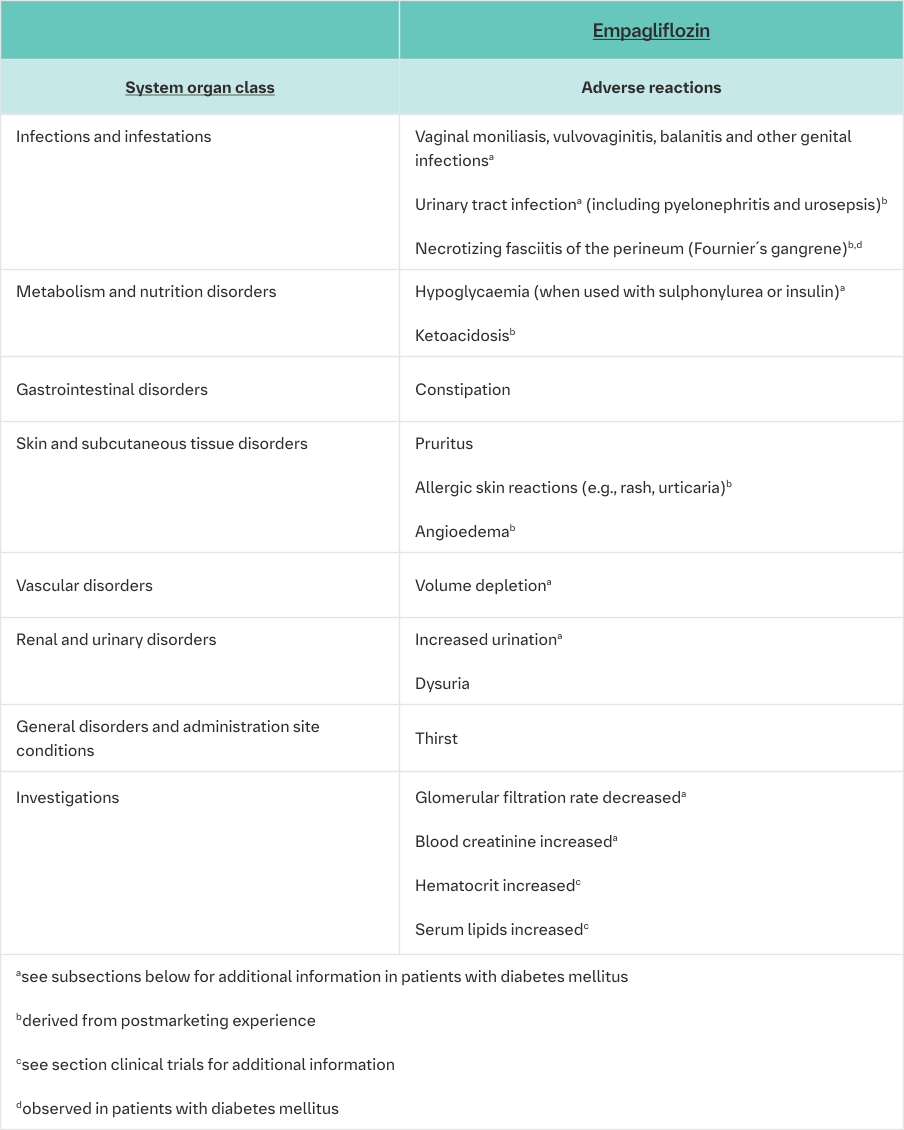
Description of selected adverse reactions
The frequencies below are calculated for adverse reactions regardless of causality.
Hypoglycaemia
The frequency of hypoglycaemia depended on the background therapy in the respective studies and was similar for Empagliflozin (Jardiance) and placebo as monotherapy, as add-on to metformin, as add-on to pioglitazone +/- metformin, and as add-on with linagliptin + metformin. The frequency of patients with hypoglycaemia was increased in patients treated with Empagliflozin (Jardiance) compared to placebo when given as add-on to metformin plus sulfonylurea, and as add-on to insulin +/- metformin and +/-sulfonylurea. (see section Dosage and Administration; see table below).
Major hypoglycaemia (events requiring assistance)
The frequency of patients with major hypoglycaemic events was low (<1%) and similar for Empagliflozin (Jardiance) and placebo as monotherapy, as add-on to metformin +/- sulfonylurea, as add-on to pioglitazone +/- metformin, and as add-on with linagliptin + metformin.
The frequency of patients with major hypoglycaemic events was increased in patients treated with Empagliflozin (Jardiance) compared to placebo when given as add-on to insulin +/- metformin and +/-sulfonylurea.
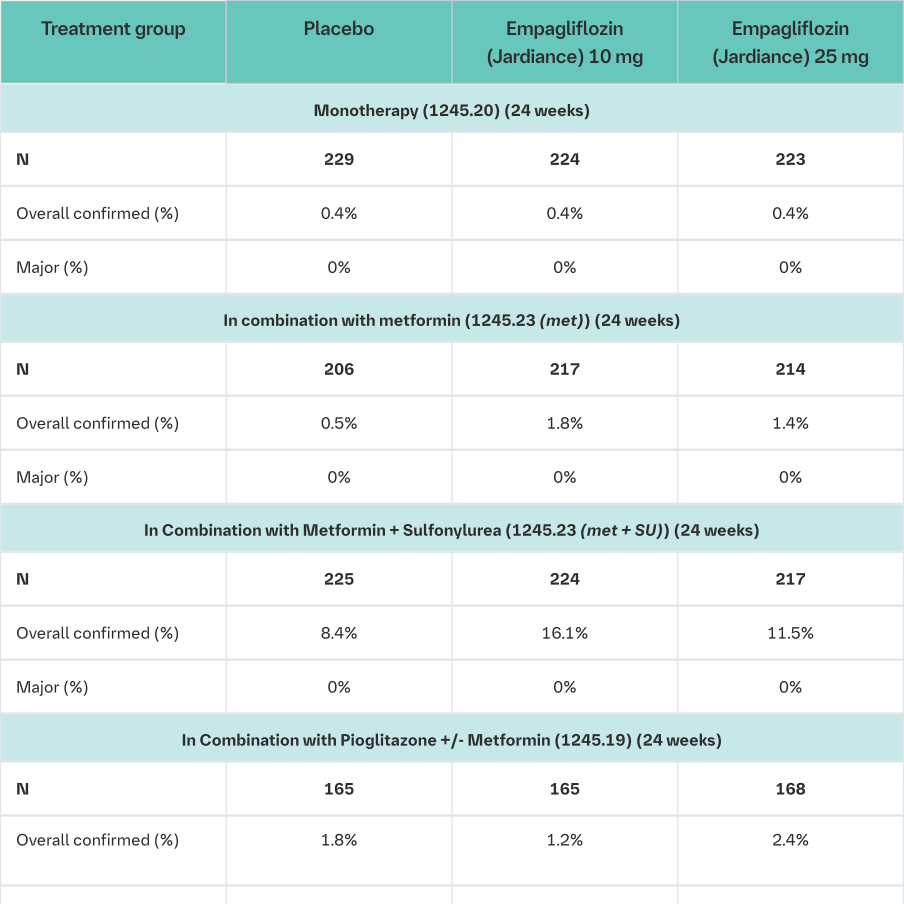
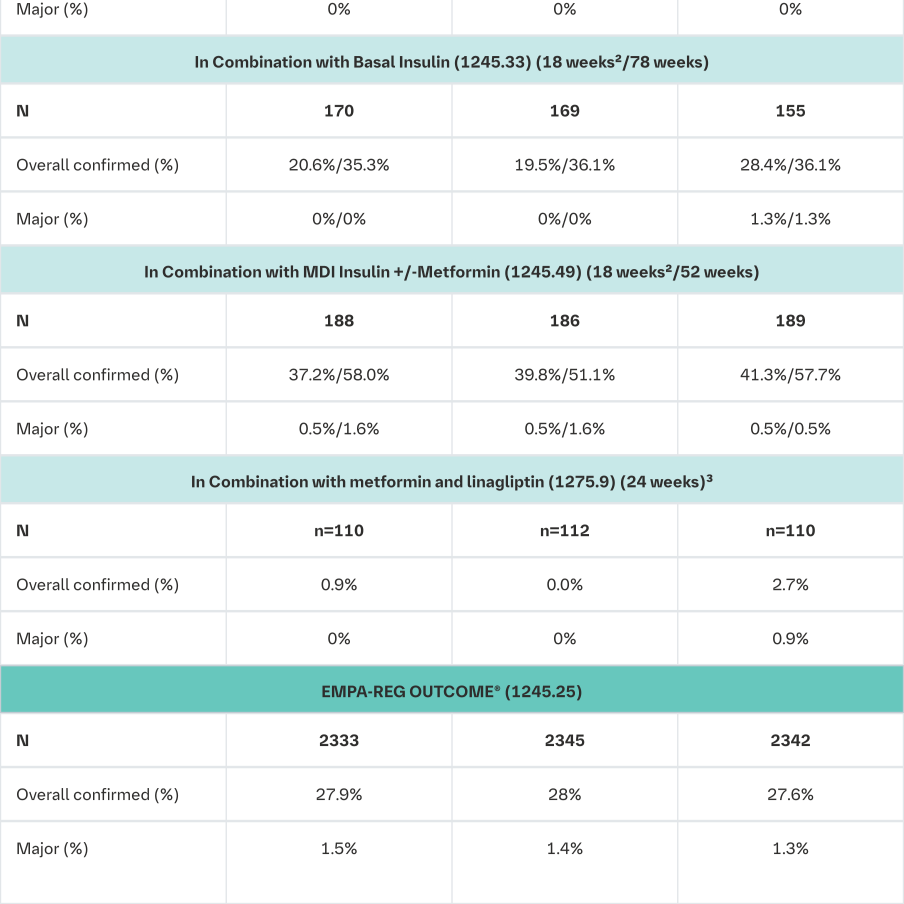
Confirmed: blood glucose ≤70 mL/dL or required assistance Major: required assistance
1i.e. patients who had received at least one dose of study drug
2The dose of insulin as background medication was to be stable for the first 18 weeks
3This was a fixed-dose combination of empagliflozin with linagliptin 5 mg with a background treatment with metformin. (see also Clinical Trials section)
Source data: 1245.19 [U12-1516, Table 15.3.2.3: 3], 1245.20 [c01950507-04, Table 15.3.2.3: 2], 1245.23 [U12-1518, Tables 15.1.3.2.3: 3 and 15.2.3.2.3: 3], 1245.33 [U12-3817, Tables 15.3.2.3: 3 and 15.4.5: 3], 1245.49 [U13-2122, Tables 15.3.2.4: 3 and 15.3.2.5: 3], 1275.9 [c02820144-02 Table 15.3.1.3: 6] 1245.25 [c02695839-01, Table 15.3.1.4:4]
Urinary tract infection
The overall frequency of urinary tract infection adverse events was similar in patients treated with Empagliflozin (Jardiance) 25 mg and placebo (7.0% and 7.2%), and higher in patients treated with Empagliflozin (Jardiance) 10 mg (8.8%). Similar to placebo, urinary tract infection was reported more frequently for Empagliflozin (Jardiance) in patients with a history of chronic or recurrent urinary tract infections. The intensity of urinary tract infections was similar to placebo for mild, moderate, and severe intensity reports. Urinary tract infection events were reported more frequently for empagliflozin compared to placebo in female patients, but not in male patients.
Vaginal moniliasis, vulvovaginitis, balanitis and other genital infection
Vaginal moniliasis, vulvovaginitis, balanitis and other genital infections were reported more frequently for Empagliflozin (Jardiance) 10 mg (4.0%) and Empagliflozin (Jardiance) 25 mg (3.9%) compared to placebo (1.0%), and were reported more frequently for empagliflozin compared to placebo in female patients, and the difference in frequency was less pronounced in male patients. The genital tract infections were mild and moderate in intensity, none was severe in intensity.
Increased urination
As expected via its mechanism of action, increased urination (as assessed by PT search including pollakiuria, polyuria, nocturia) was observed at higher frequencies in patients treated with Empagliflozin (Jardiance) 10 mg (3.5%) and Empagliflozin (Jardiance) 25 mg (3.3%) compared to placebo (1.4%). Increased urination was mostly mild or moderate in intensity. The frequency of reported nocturia was comparable between placebo and Empagliflozin (Jardiance) (<1%).
Volume depletion
The overall frequency of volume depletion (including the predefined terms blood pressure (ambulatory) decreased, blood pressure systolic decreased, dehydration, hypotension, hypovolaemia, orthostatic hypotension, and syncope) was similar to placebo (Empagliflozin (Jardiance) 10 mg 0.6%, Empagliflozin (Jardiance) 25 mg 0.4% and placebo 0.3%). The effect of empagliflozin on urinary glucose excretion is associated with osmotic diuresis, which could affect hydration status of patients aged 75 years and older. In patients ≥75 years of age (pooling of all patients with diabetes, n=13,402) the frequency of volume depletion events was similar for Empagliflozin (Jardiance) 10 mg (2.3%) compared to placebo (2.1%), but it increased with Empagliflozin (Jardiance) 25 mg (4.3%).
Blood creatinine increased and glomerular filtration rate decreased
The overall frequency of patients with increased blood creatinine and decreased glomerular filtration rate was similar between empagliflozin and placebo (blood creatinine increased: empagliflozin 10 mg 0.6%, empagliflozin 25 mg 0.1%, placebo 0.5%; glomerular filtration rate decreased: empagliflozin 10 mg 0.1%, empagliflozin 25 mg 0%, placebo 0.3%).
In placebo-controlled, double-blind studies up to 76 weeks, initial transient increases in creatinine (mean change from baseline after 12 weeks: empagliflozin 10 mg 0.02 mg/dL, empagliflozin 25 mg 0.01 mg/dL) and initial transient decreases in estimated glomerular filtration rates (mean change from baseline after 12 weeks: empagliflozin 10 mg -1.34 mL/min/ 1.73 m2, empagliflozin 25 mg -1.37 mL/min/1.73 m2) have been observed. These changes were generally reversible during continuous treatment or after drug discontinuation (see section Clinical Trials figure 6 for the eGFR course in the EMPA-REG outcome® study).
10. Overdose
During controlled clinical trials in healthy subjects, single doses of up to 800 mg empagliflozin were well tolerated.
Therapy
In the event of an overdose, supportive treatment should be initiated as appropriate to the patient’s clinical status. The removal of empagliflozin by haemodialysis has not been studied.
11. Pharmacological properties
Pharmacotherapeutic group: SGLT2 Inhibitor, ATC code: A10BK03.
Mode of action
Empagliflozin is a reversible, highly potent and selective competitive inhibitor of SGLT2 with an IC50 of 1.3 nM. It has a 5000-fold selectivity over human SGLT1 (IC50 of 6278 nM), responsible for glucose absorption in the gut. Furthermore high selectivity could be shown toward other glucose transporters (GLUTs) responsible for glucose homeostasis in the different tissues.
SGLT2 is highly expressed in the kidney, whereas expression in other tissues is absent or very low. It is responsible as the predominant transporter for reabsorption of glucose from the glomerular filtrate back into the circulation. In patients with type 2 diabetes mellitus (T2DM) and hyperglycaemia a higher amount of glucose is filtered and reabsorbed.
Empagliflozin improves glycaemic control in patients with T2DM by reducing renal glucose reabsorption. The amount of glucose removed by the kidney through this glucuretic mechanism is dependent upon the blood glucose concentration and GFR. Through inhibition of SGLT2 in patients with T2DM and hyperglycaemia, excess glucose is excreted in the urine.
In patients with T2DM, urinary glucose excretion increased immediately following the first dose of empagliflozin and is continuous over the 24-hour dosing interval. Increased urinary glucose excretion was maintained at the end of 4-week treatment period, averaging approximately 78 g/day with empagliflozin 25 mg once daily. Increased urinary glucose excretion resulted in an immediate reduction in plasma glucose levels in patients with T2DM.
Empagliflozin (10 mg and 25 mg) improves both fasting and post-prandial plasma glucose levels.
The mechanism of action of empagliflozin is independent of beta cell function and insulin pathway, and this contributes to a low risk of hypoglycaemia. Improvement of surrogate markers of beta cell function including Homeostasis Model Assessment-B (HOMA-β) and proinsulin to insulin ratio were noted. In addition, urinary glucose excretion triggers calorie loss, associated with body fat loss and body weight reduction.
The glucosuria observed with empagliflozin is accompanied by mild diuresis which may contribute to sustained and moderate reduction of blood pressure.
Empagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to: increasing tubuloglomerular feedback and reducing intraglomerular pressure, lowering both pre- and afterload of the heart, downregulating sympathetic activity and reducing left ventricular wall stress as evidenced by lower NT-proBNP values which may have beneficial effects on cardiac remodeling, filling pressures and diastolic function as well as preserving kidney structure and function. Other effects such as an increase in haematocrit, a reduction in body weight and blood pressure may further contribute to the beneficial cardial and renal effects.
Clinical trials
Type 2 diabetes mellitus
A total of 17331 patients with type 2 diabetes were evaluated in 15 double-blind, placebo- and active-controlled clinical studies, of which 4603 patients received empagliflozin 10 mg and 5567 received empagliflozin 25 mg. Six studies had a treatment duration of 24 weeks; in extensions of applicable studies, and other trials, patients were exposed to Empagliflozin (Jardiance) for up to 102 weeks.
Treatment with empagliflozin (10 mg and 25 mg) as monotherapy and in combination with metformin, pioglitazone, sulfonylurea, DPP-4 inhibitors, and insulin lead to clinically relevant improvements in HbA1c, fasting plasma glucose (FPG), body weight, systolic and diastolic blood pressure (SBP and DBP, respectively). Administration of empagliflozin 25 mg resulted in a higher proportion of patients achieving an HbA1c goal of <7% and fewer patients needing glycaemic rescue compared to empagliflozin 10 mg and placebo. There was a clinically meaningful improvement in HbA1c in all subgroups of gender, race, geographic region, time since diagnosis of T2DM, body mass index, insulin resistance based on HOMA-IR, and beta cell function based on HOMA-β. Higher baseline HbA1c was associated with a greater reduction in HbA1c. Clinically meaningful HbA1c reduction was seen for patients with eGFR >30 mL/min/1.73 m² (See Dosage and administration, Patients with renal impairment). In patients aged 75 years and older, reduced efficacy of Empagliflozin (Jardiance) was observed.
Empagliflozin as monotherapy
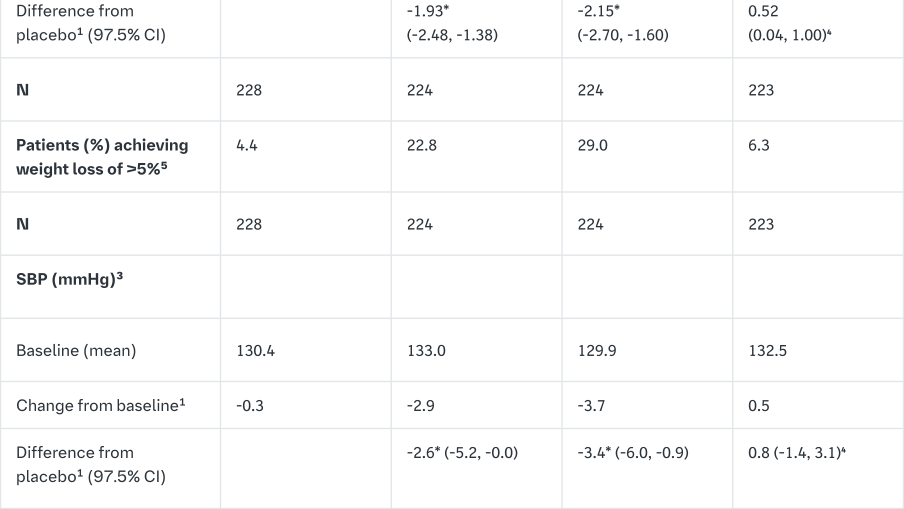
The efficacy and safety of empagliflozin (10 mg and 25 mg) as monotherapy was evaluated in a double-blind, placebo- and active-controlled study of 24 weeks duration in treatment-naïve patients. Treatment with Empagliflozin (Jardiance) resulted in statistically significant reductions in HbA1c, body weight and SBP compared to placebo (Table 3) and a clinically meaningful decrease in FPG. A numerical decrease in DPB was seen but did not reach statistical significance versus placebo (-1.0 mmHg for empagliflozin 10 mg, -1.9 mmHg for empagliflozin 25 mg, -0.5 for placebo, and +0.7 mmHg for sitagliptin).
In a prespecified analysis of patients (N=201) with a baseline HbA1c ≥8.5% to ≤10%, empagliflozin resulted in a reduction in HbA1c from baseline of -1.44% for empagliflozin 10 mg, -1.43% for empagliflozin 25 mg, +0.01% for placebo, and -1.04% for sitagliptin.
In the double-blind, placebo-controlled extension of this study, reductions of HbA1c (change from baseline of -0.65% for empagliflozin 10 mg, -0.76% for empagliflozin 25 mg, +0.13% for placebo, and -0.53% for sitagliptin), body weight (change from baseline of -2.24 kg for empagliflozin 10 mg, -2.45 kg for empagliflozin 25 mg, -0.43 kg for placebo, and +0.10 kg for sitagliptin) and blood pressure (SBP: change from baseline of, -4.1 mmHg for empagliflozin 10 mg, -4.2 mmHg for empagliflozin 25 mg, -0.7 mmHg for placebo, and -0.3 mmHg for sitagliptin, DBP: change from baseline of -1.6 mmHg for empagliflozin 10 mg, -1.6 mmHg for empagliflozin 25 mg, -0.6 mmHg for placebo, and -0.1 mmHg for sitagliptin) were sustained up to Week 76.
Treatment with Empagliflozin (Jardiance) daily significantly improved marker of beta cell function HOMA-B.
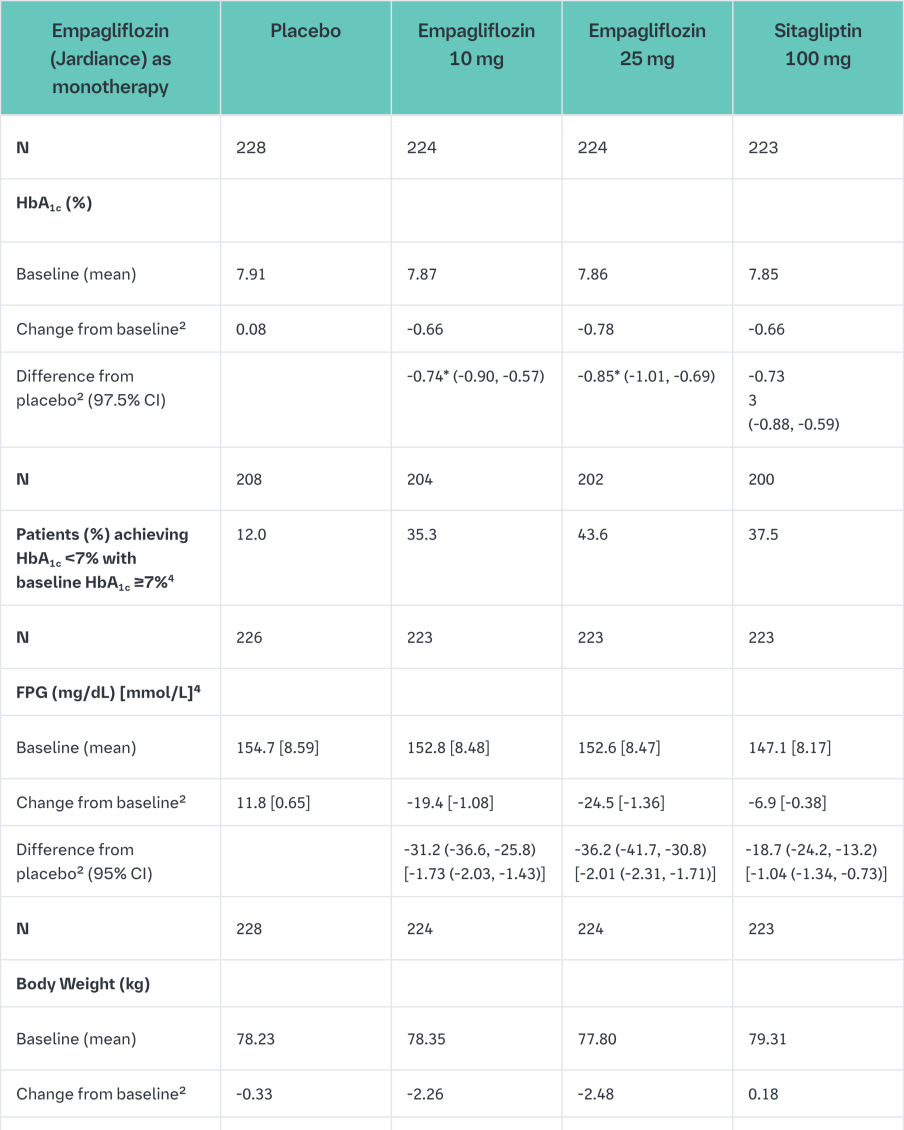
1Last observation (prior to glycaemic rescue) carried forward (LOCF)
2mean adjusted for baseline value and stratification
3Last observation (prior to glycaemic rescue or antihypertensive rescue) carried forward (LOCF)
495% CI
5not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
* <0.0001
Empagliflozin as add on to metformin therapy
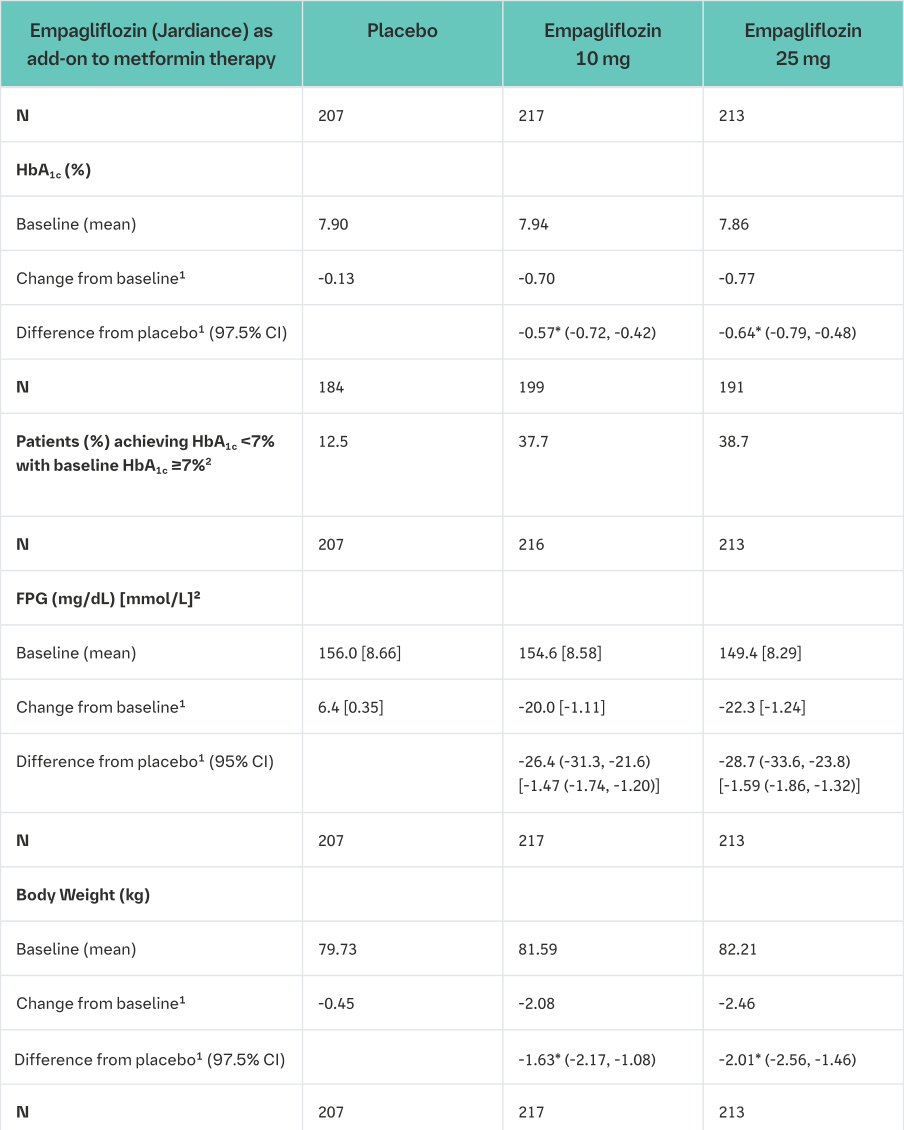
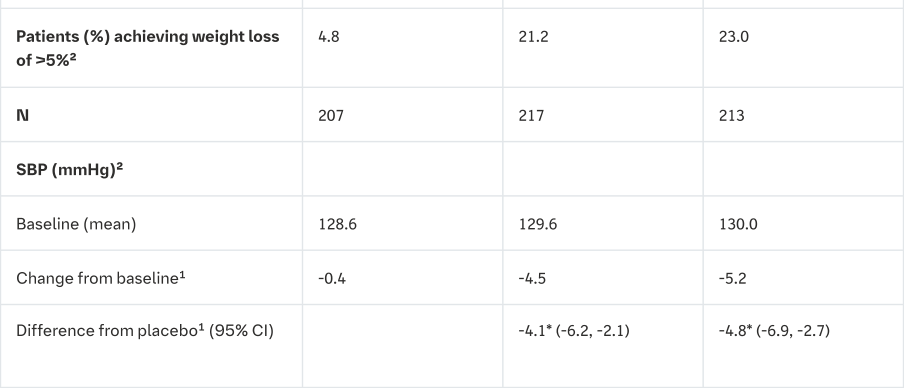
A double-blind, placebo-controlled study of 24 weeks duration was conducted to evaluate the efficacy and safety of empagliflozin in patients not sufficiently treated with metformin.
Treatment with Empagliflozin (Jardiance) resulted in statistically significant improvements in HbA1c and body weight, and clinically meaningful reductions in FPG and blood pressure compared to placebo (Table 4).
In the double-blind placebo-controlled extension of this study, reductions of HbA1c (change from baseline of -0.62% for empagliflozin 10 mg, -0.74% for empagliflozin 25 mg and -0.01% for placebo), body weight (change from baseline of -2.39 kg for empagliflozin 10 mg, -2.65 kg for empagliflozin 25 mg and -0.46 kg for placebo) and blood pressure (SBP: change from baseline of -5.2 mmHg for empagliflozin 10 mg, -4.5 mmHg for empagliflozin 25 mg and 0.8 mmHg for placebo, DBP: change from baseline of -2.5 mmHg for empagliflozin 10 mg, -1.9 mmHg for empagliflozin 25 mg and -0.5 mmHg for placebo) were sustained up to Week 76.
1mean adjusted for baseline value and stratification
2not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
3Last observation (prior to glycaemic rescue) carried forward (LOCF)
*p-value <0.0001
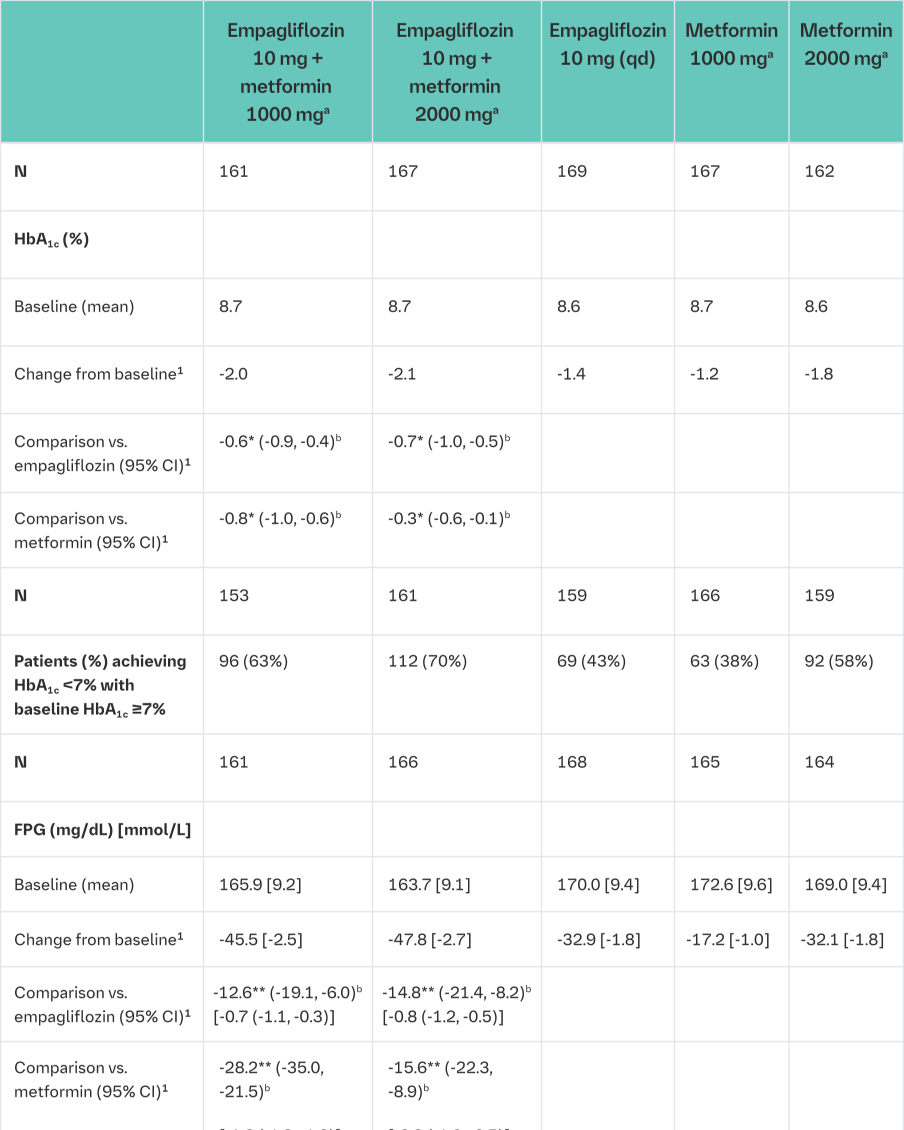
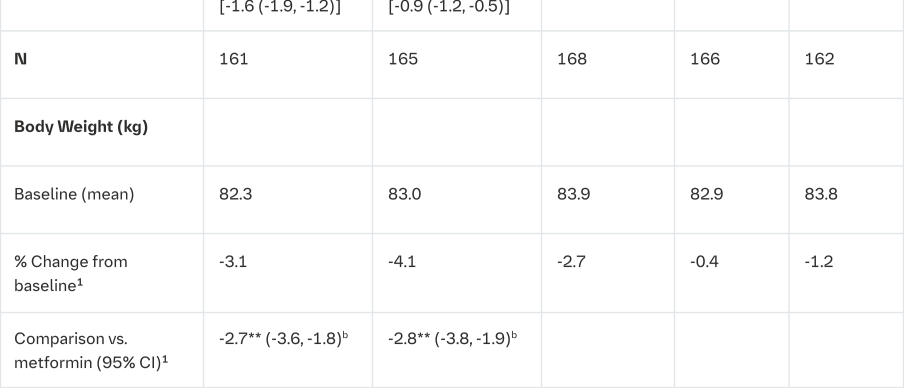
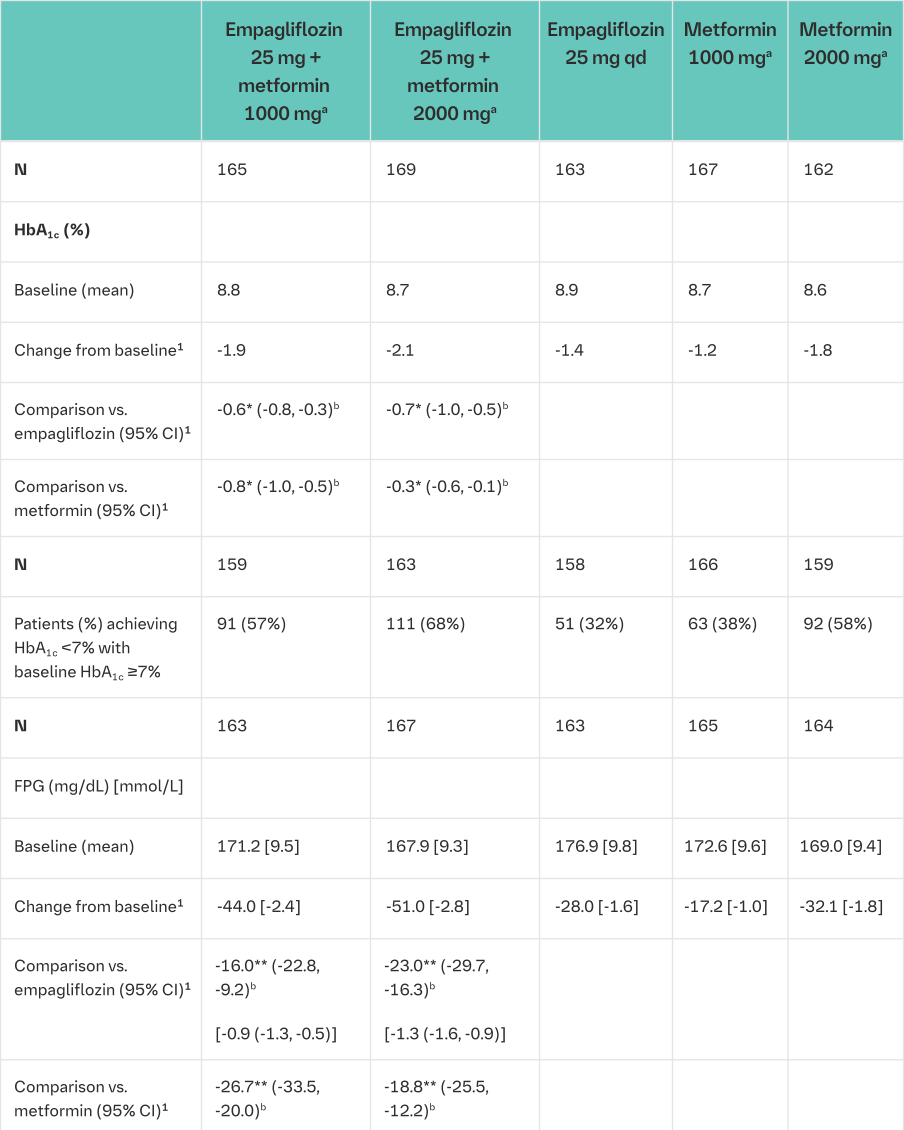
Empagliflozin and metformin combination therapy in drug-naïve patients
A factorial design study of 24 weeks duration was conducted to evaluate the efficacy and safety of empagliflozin in drug-naïve patients. Treatment with empagliflozin in combination with metformin (5 mg and 500 mg; 5 mg and 1000 mg; 12.5 mg and 500 mg, and 12.5 mg and 1000 mg given twice daily) provided statistically significant improvements in HbA1c and led to significantly greater reductions in FPG and body weight compared to the individual components. A greater proportion of patients with a baseline HbA1c ≥7.0% and treated with empagliflozin in combination with metformin achieved a target HbA1c <7% compared to the individual components (Tables 5 and 6).
aGiven in two equally divided doses per day
bFull analysis population (observed case) using MMRM. MMRM model included treatment, renal function, region, visit, visit by treatment interaction, and baseline HbA1c; FPG included baseline FPG in addition; weight included baseline weight in addition
1mean adjusted for baseline value
2Analyses were performed on the full analysis set (FAS) using an observed cases (OC) approach
*p≤0.0062 for HbA1c
**Analysis in an exploratory manner: p≤0.0002 for FPG and p<0.0001 for body weight
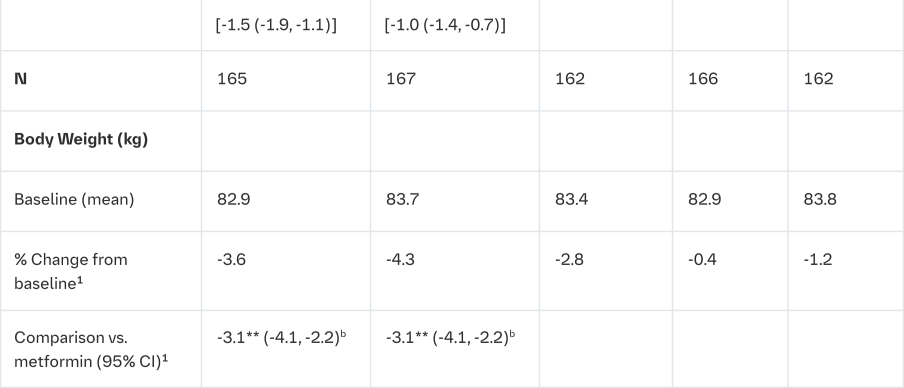
aGiven in two equally divided doses per day
bFull analysis population (observed case) using MMRM. MMRM model included treatment, renal function, region, visit, visit by treatment interaction, and baseline HbA1c; FPG included baseline FPG in addition; weight included baseline weight in addition
1mean adjusted for baseline value
2Analyses were performed on the full analysis set (FAS) using an observed cases (OC) approach
*p≤0.0056 for HbA1c
**Analysis in an exploratory manner: p<0.0001 for FPG and p<0.0001 for body weight
Empagliflozin as add on to a combination of metformin and sulphonylurea therapy
A double-blind, placebo-controlled study of 24 weeks duration was conducted to evaluate the efficacy and safety of empagliflozin in patients not sufficiently treated with a combination of metformin and a sulphonylurea. Treatment with Empagliflozin (Jardiance) resulted in statistically significant improvements in HbA1c and body weight and clinically meaningful reductions in FPG and blood pressure compared to placebo (Table 7).
In the double-blind, placebo-controlled extension of this study, reductions of HbA1c (change from baseline of -0.74% for empagliflozin 10 mg, -0.72% for empagliflozin 25 mg and -0.03% for placebo), body weight (change from baseline of -2.44 kg for empagliflozin 10 mg, -2.28 kg for empagliflozin 25 mg and -0.63 kg for placebo) and blood pressure (SBP: change from baseline of -3.8 mmHg for empagliflozin 10 mg, -3.7 mmHg for empagliflozin 25 mg and -1.6 mmHg for placebo, DBP: change from baseline of -2.6 mmHg for empagliflozin 10 mg, -2.3 mmHg for empagliflozin 25 mg and -1.4 mmHg for placebo) were sustained up to Week 76.
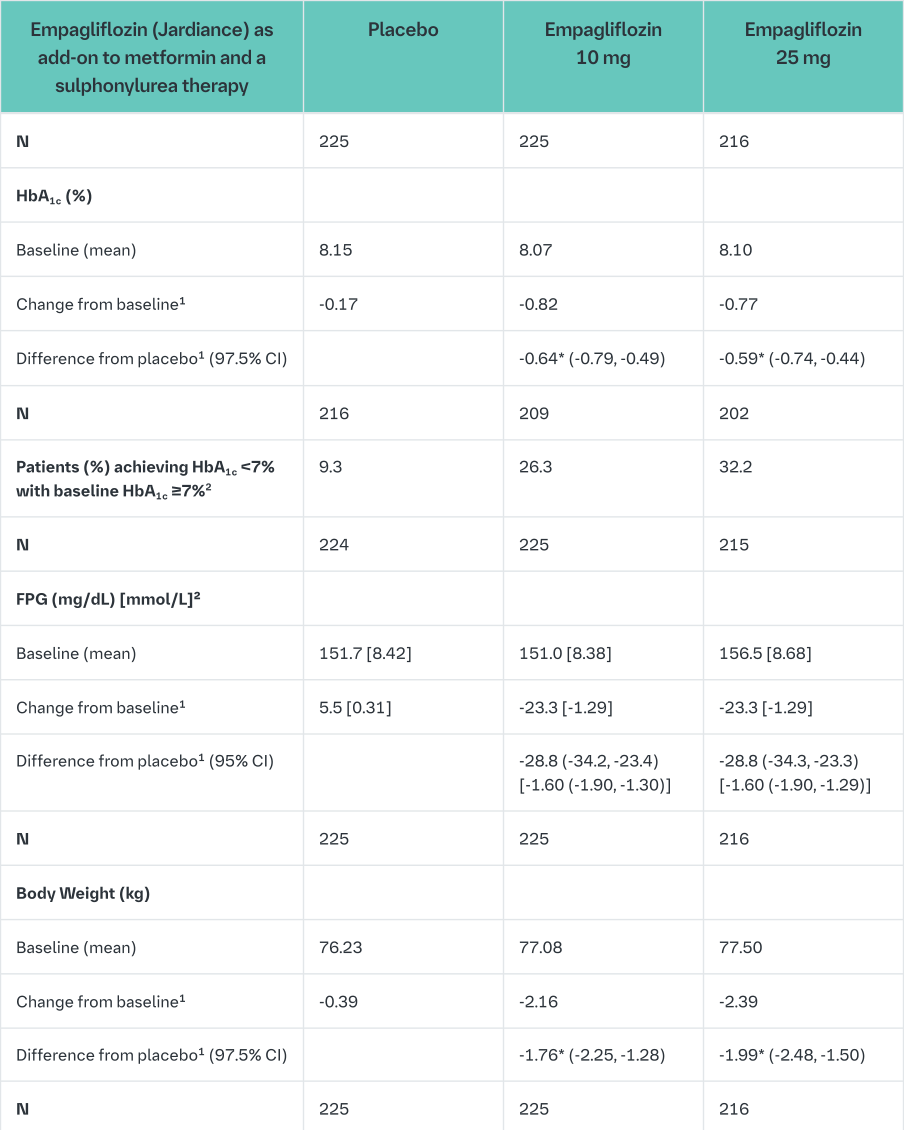
1mean adjusted for baseline value and stratification
2not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
3Last observation (prior to glycaemic rescue) carried forward (LOCF)
*p-value <0.0001
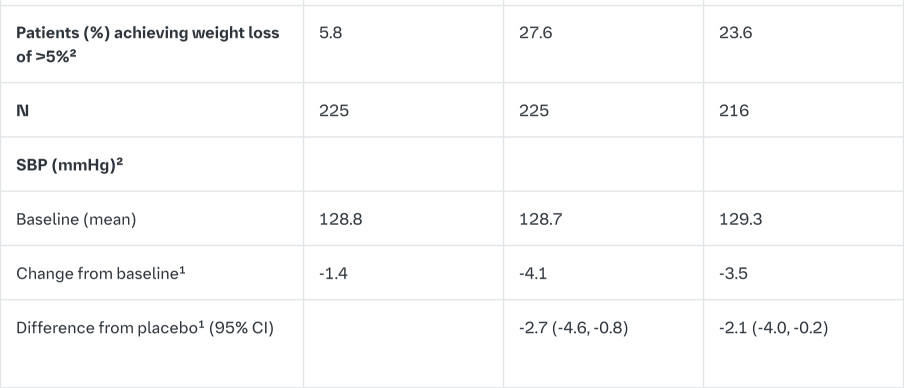
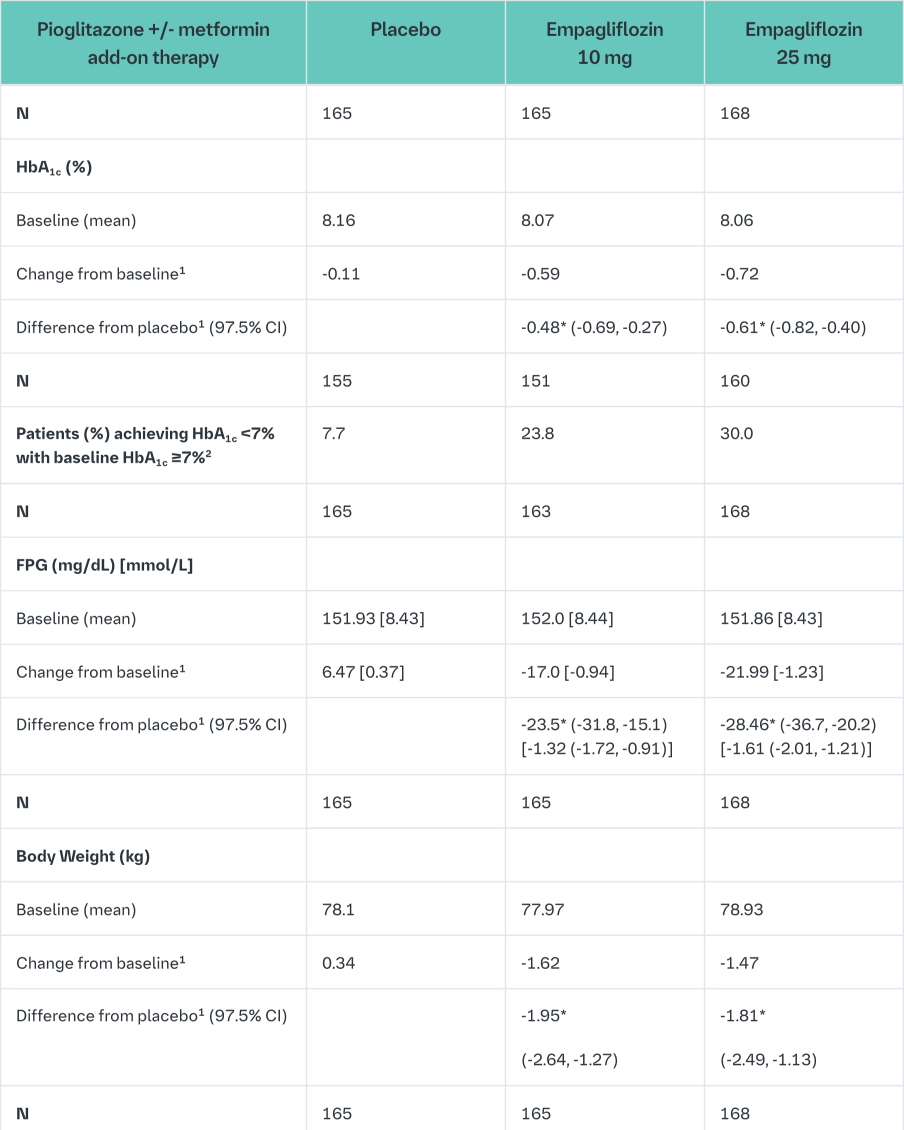
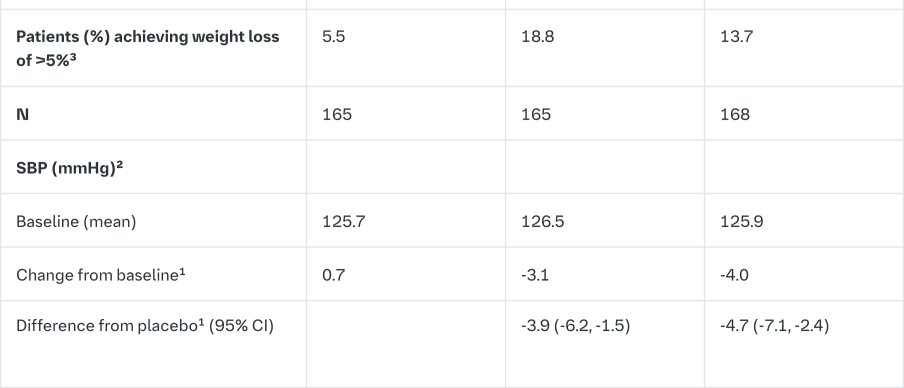
Empagliflozin as add on to a combination of pioglitazone therapy (+/- metformin)
The efficacy and safety of empagliflozin was evaluated in a double-blind, placebo-controlled study of 24 weeks duration in patients not sufficiently treated with a combination of metformin and pioglitazone or pioglitazone alone. Empagliflozin in combination with pioglitazone (dose ≥30 mg) with or without metformin resulted in statistically significant reductions in HbA1c, FPG, and body weight and clinically meaningful reductions in blood pressure compared to placebo (Table 8).
In the double-blind, placebo-controlled extension of this study, reductions of HbA1c (change from baseline of -0.61% for empagliflozin 10 mg, -0.70% for empagliflozin 25 mg and -0.01% for placebo), body weight (change from baseline of -1.47 kg for empagliflozin 10 mg, -1.21 kg for empagliflozin 25 mg and +0.50 kg for placebo) and blood pressure (SBP: change from baseline of -1.7 mmHg for empagliflozin 10 mg, -3.4 mmHg for empagliflozin 25 mg and +0.3 mmHg for placebo, DBP: change from baseline of -1.3 mmHg for empagliflozin 10 mg, -2.0 mmHg for empagliflozin 25 mg and +0.2 mmHg for placebo) were sustained up to Week 76.
1mean adjusted for baseline value and stratification
2not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
3Last observation (prior to glycaemic rescue) carried forward (LOCF)
*p-value <0.0001
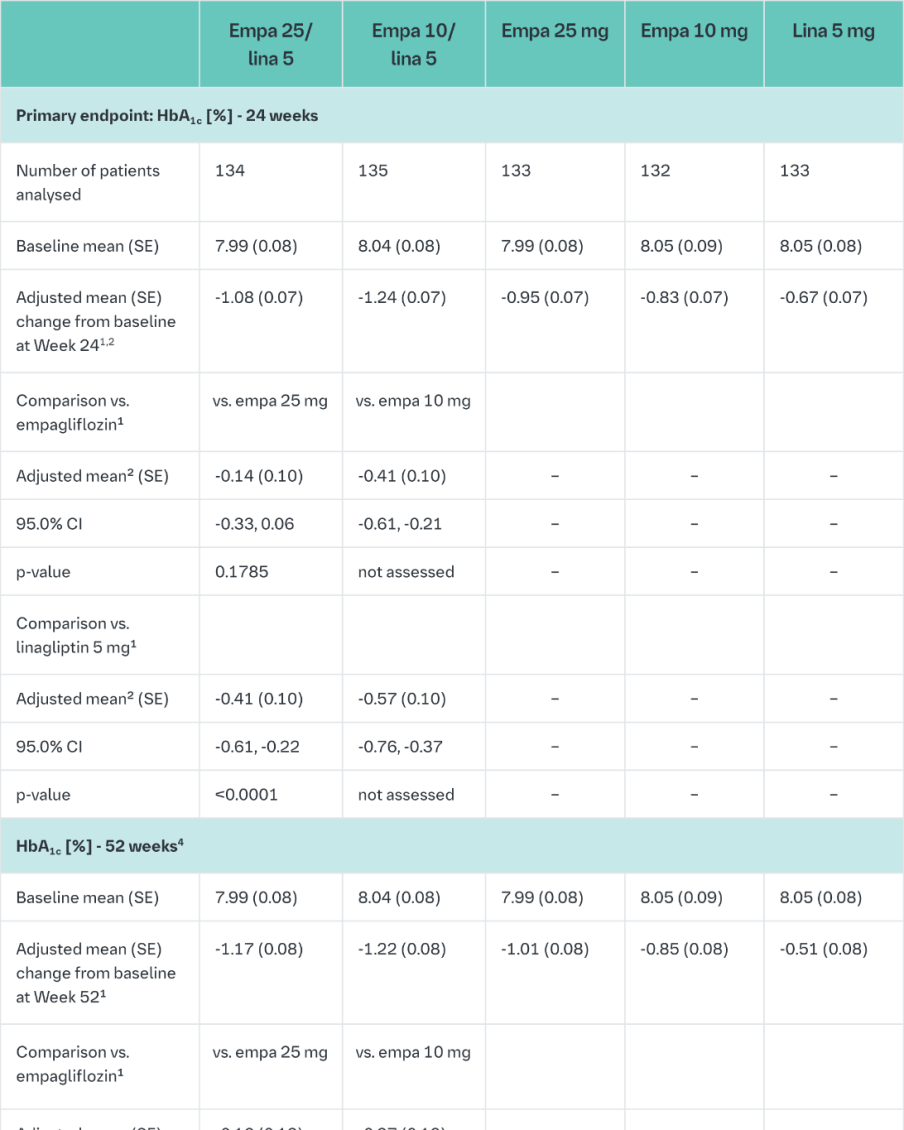
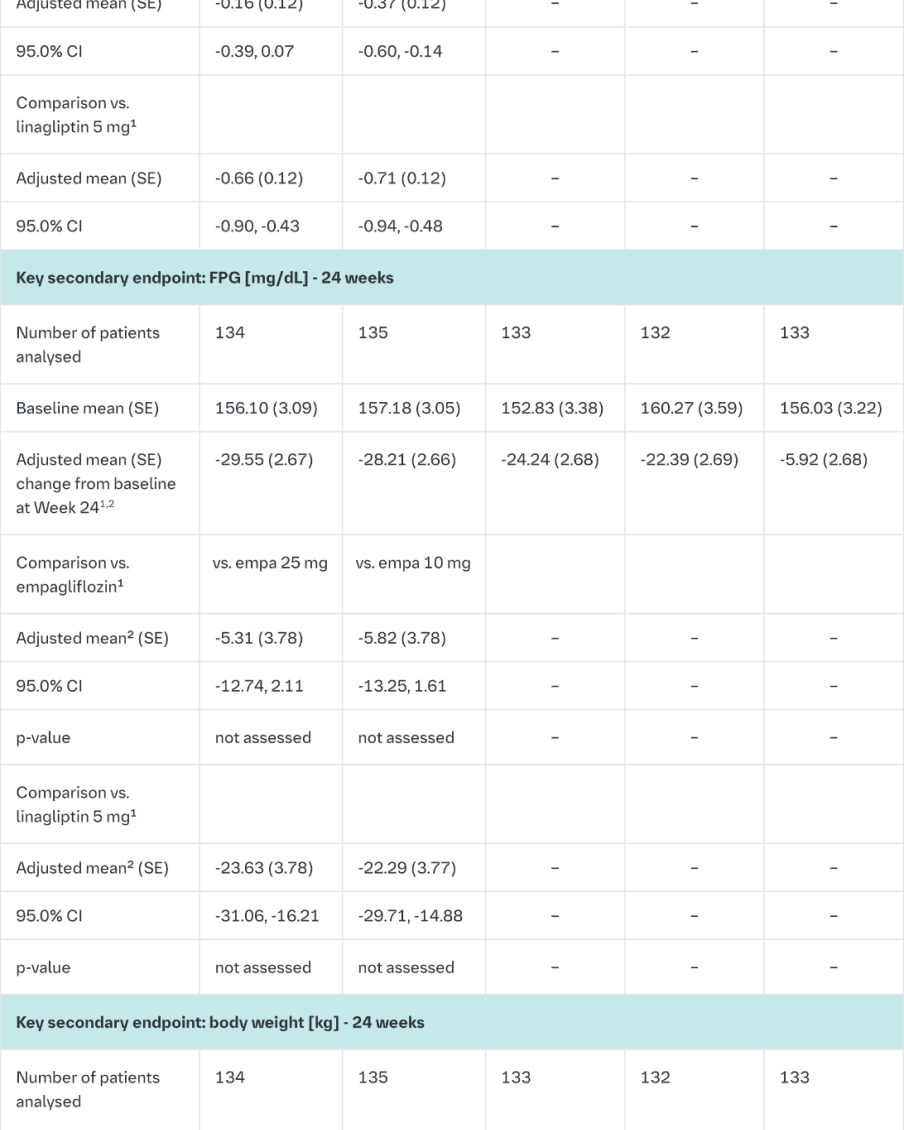
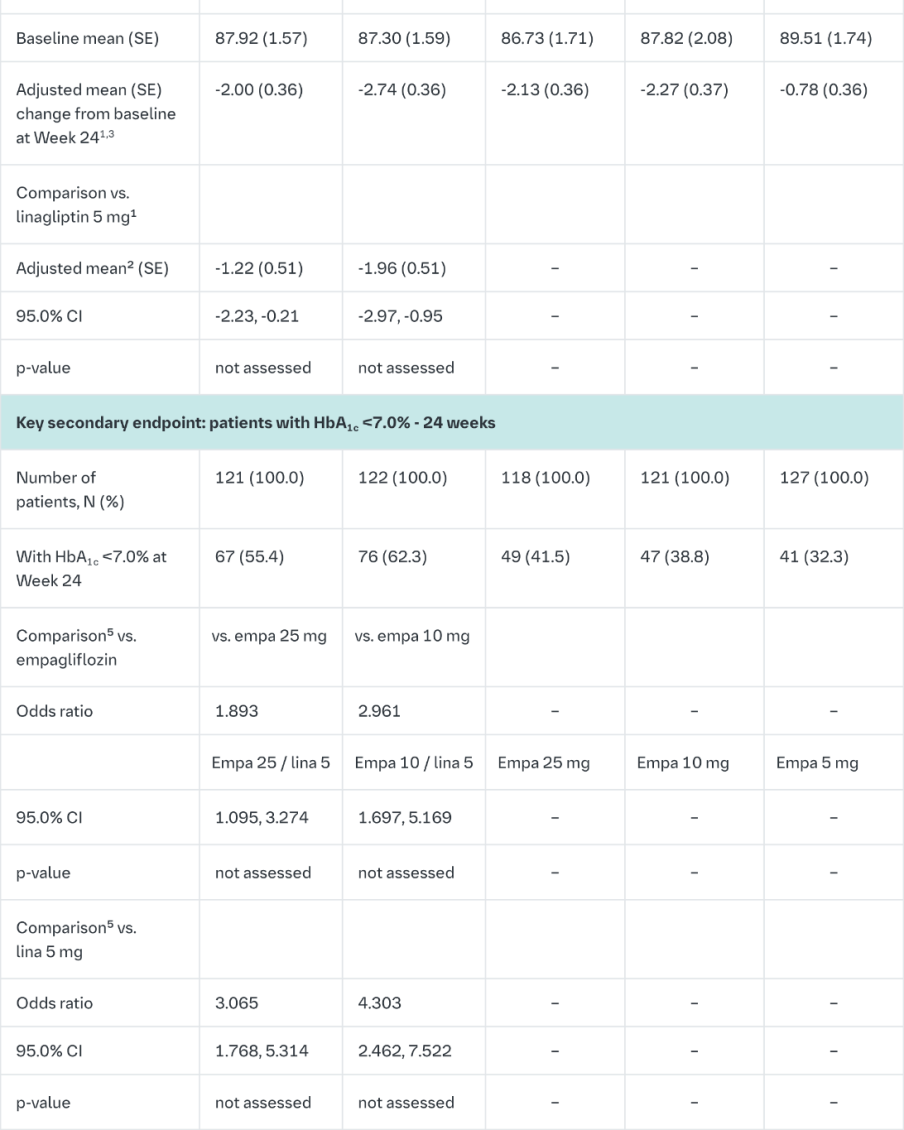
Empagliflozin and linagliptin in treatment-naïve patients
After 24-weeks treatment, empagliflozin 25 mg/linagliptin 5 mg in treatment-naïve patients provided statistically significant improvement in HbA1c compared to linagliptin 5 mg but there was no statistically significant difference between the FDC empagliflozin 25 mg/linagliptin 5 mg and empagliflozin 25 mg (Table 9). Compared to linagliptin 5 mg, both doses of the empagliflozin/linagliptin FDC provided statistically relevant improvements in body weight. After 24 weeks’ treatment with empagliflozin/linagliptin, both SBPs and DBPs were reduced, -2.9/-1.1 mmHg (n.s. versus linagliptin 5 mg for SBP and DBP) for empagliflozin 25 mg/linagliptin 5 mg and -3.6/-0.7 mmHg (p<0.05 versus linagliptin 5 mg for SBP, n.s. for DBP) for empagliflozin 10 mg/linagliptin 5 mg. Rescue therapy was used in 2 (1.5%) patients treated with empagliflozin 25 mg/linagliptin 5 mg and in 1 (0.7%) patient treated with empagliflozin 10 mg/linagliptin 5 mg compared to 11 (8.3%) patients treated with linagliptin 5 mg, 1 (0.8%) patients treated with empagliflozin 25 mg and 4 (3.0%) patients treated with empagliflozin 10 mg. Clinically meaningful reductions in HbA1c (Table 9) and SBPs were observed at week 52, -2.0 mmHg (n.s. versus linagliptin 5 mg) for empagliflozin 25 mg/linagliptin 5 mg and -1.7 mmHg (n.s. versus linagliptin 5 mg) for empagliflozin 10 mg/linagliptin 5 mg.
1Last observation (prior to glycaemic rescue) carried forward (LOCF)
2Mean adjusted for baseline value and stratification
3ANCOVA model includes baseline body weight, baseline HbA1c, baseline eGFR (MDRD), geographical region, and treatment; based on FAS (LOCF). The comparisons vs. empa were exploratory and not part of the testing hierarchy (empa 25 mg/lina 5 mg vs. empa 25 mg: adjusted mean 0.19 (95% CI 0.65, 1.03) kg; empa 10 mg/lina 5 mg vs. empa 10 mg: 0.07 (0.91, 0.77) kg)
4Not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints. Specification ‘not assessed’ means that the previous hierarchical test in the confirmatory sequence failed so no subsequent testing was performed
5Logistic regression includes baseline HbA1c, baseline eGFR (MDRD), geographical region, and treatment; based on FAS (NCF), patients with HbA1c of 7% and above at baseline
In a prespecified subgroup of patients with baseline HbA1c greater or equal than 8.5% the reduction from baseline in HbA1c with empagliflozin 25 mg/linagliptin 5 mg was -1.9% at 24 weeks (p<0.0001 versus linagliptin 5 mg, n.s. versus empagliflozin 25 mg) and - 2.0% at 52 weeks (p<0.0001 versus linagliptin 5 mg, p<0.05 versus empagliflozin 25 mg) and with empagliflozin 10 mg/linagliptin 5 mg -1.9% at 24 weeks (p<0.0001 versus linagliptin 5 mg, p<0.05 versus empagliflozin 10 mg) and -2.0% at 52 weeks (p<0.0001 versus linagliptin 5 mg, p<0.05 versus empagliflozin 10 mg).
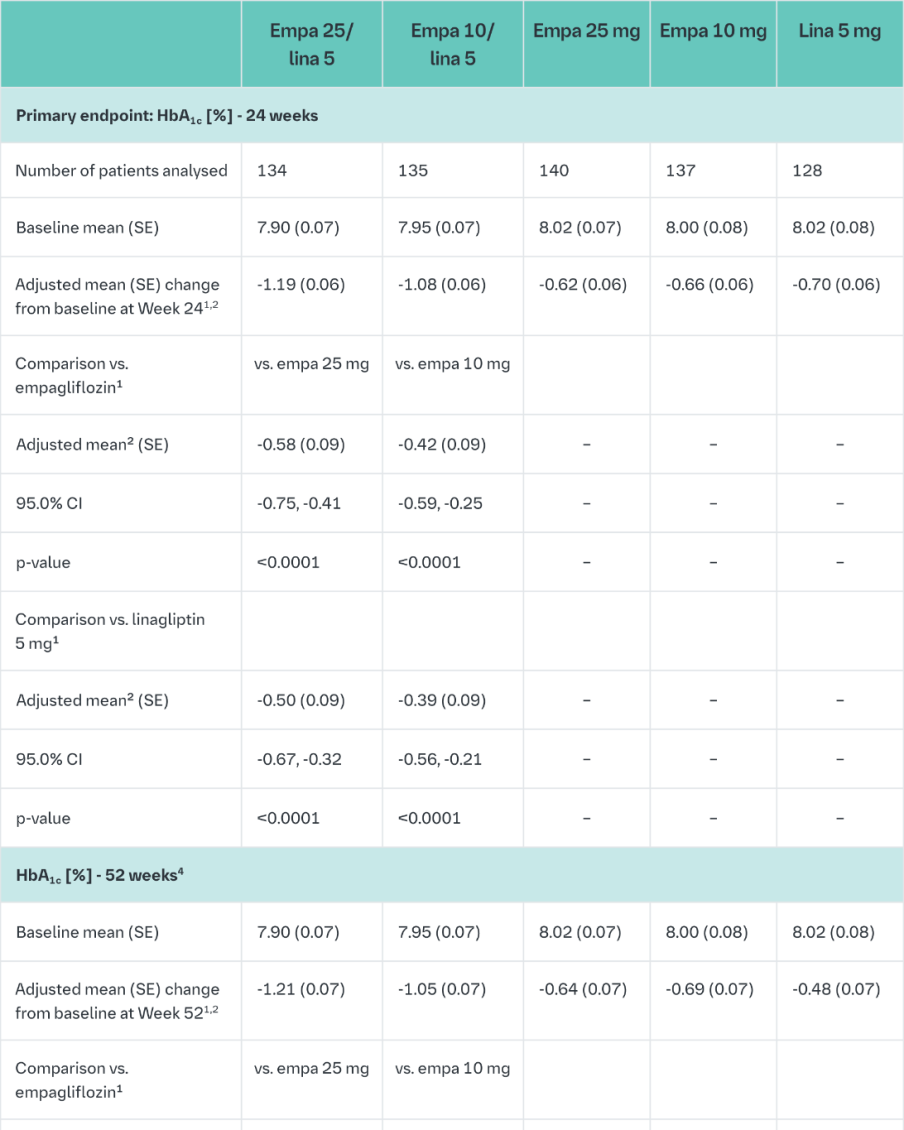
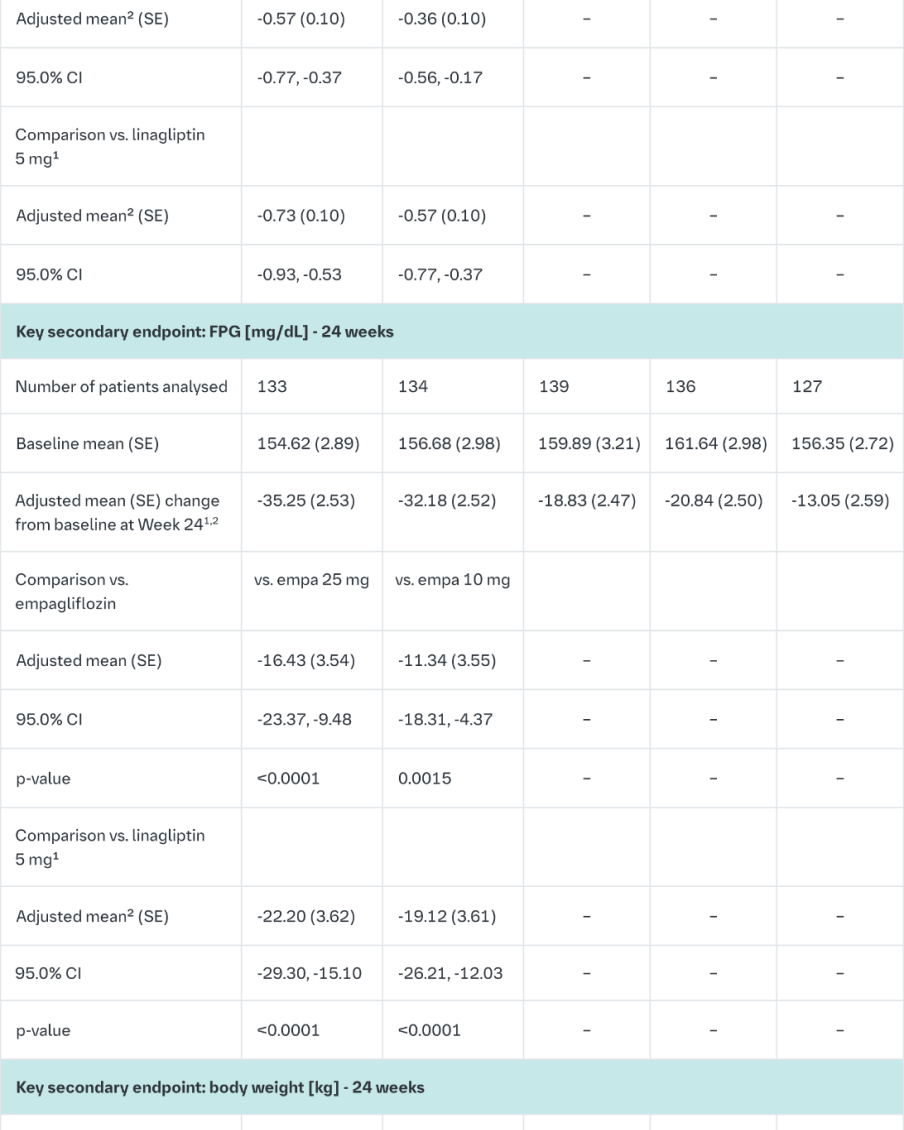
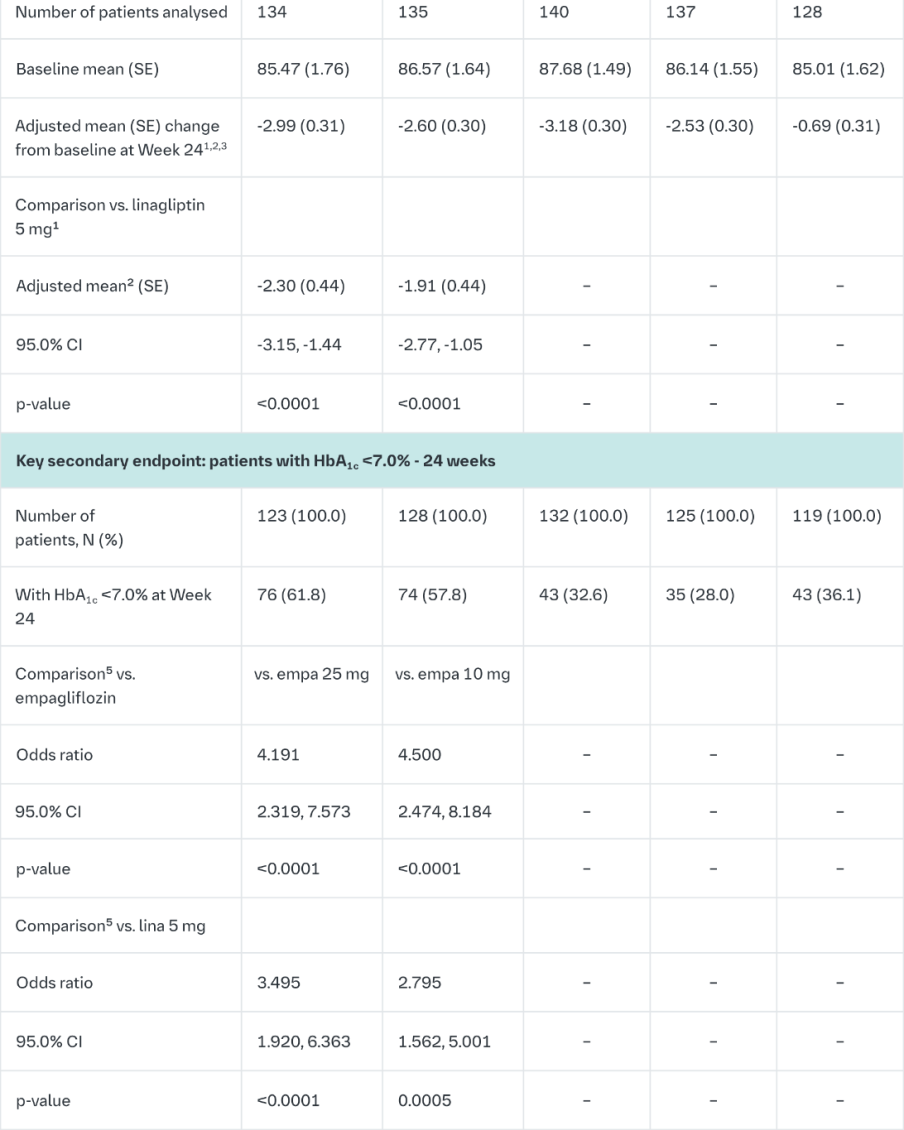
Empagliflozin and linagliptin as add-on therapy to metformin
In patients inadequately controlled on metformin 24-weeks treatment with both doses of the empagliflozin/linagliptin FDC provided statistically significant improvements in HbA1c and FPG compared to linagliptin 5 mg and also compared to empagliflozin 10 or 25 mg.
Compared to linagliptin 5 mg, both doses of the empagliflozin/linagliptin FDC provided statistically significant improvements in body weight.
A greater proportion of patients with a baseline HbA1c ≥7.0% and treated with the empagliflozin/linagliptin FDC achieved a target HbA1c of <7% compared to the individual components. (Table 10).
After 24 weeks’ treatment with empagliflozin/linagliptin, both SBPs and DBPs were reduced, -5.6/-3.6 mmHg (p<0.001 versus linagliptin 5 mg for SBP and DBP) for empagliflozin 25 mg/linagliptin 5 mg and -4.1/-2.6 mmHg (p<0.05 versus linagliptin 5 mg for SBP, n.s. for DBP) for empagliflozin 10 mg/linagliptin 5 mg. Clinically meaningful reductions in HbA1c (Table 10) and both SBPs and DBPs were observed at week 52, -3.8/-1.6 mmHg (p<0.05 versus linagliptin 5 mg for SBP and DBP) for empagliflozin 25 mg/linagliptin 5 mg and -3.1/- 1.6 mmHg (p<0.05 versus linagliptin 5 mg for SBP, n.s. for DBP) for empagliflozin 10 mg/linagliptin 5 mg.
After 24 weeks, rescue therapy was used in 1 (0.7%) patient treated with empagliflozin 25 mg/linagliptin 5 mg and in 3 (2.2%) patients treated with empagliflozin 10 mg/linagliptin 5 mg, compared to 4 (3.1%) patients treated with linagliptin 5 mg and 6 (4.3%) patients treated with empagliflozin 25 mg and 1 (0.7%) patient treated with empagliflozin 10 mg.
1Last observation (prior to glycaemic rescue) carried forward (LOCF)
2mean adjusted for baseline value and stratification
3ANCOVA model includes baseline body weight, baseline HbA1c, baseline eGFR (MDRD), geographical region, and treatment; based on FAS (LOCF). The comparisons vs. empagliflozin were exploratory and not part of the testing hierarchy (empa 25/lina 5 vs. empa 25: adjusted mean 0.19 (95% CI -0.65, 1.03) kg; empa 10/lina 5 vs. empa 10: -0.07 -0.91, 0.77) kg)
4not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
5Logistic regression includes baseline HbA1c, baseline eGFR (MDRD), geographical region, and treatment; based on FAS (NCF), patients with HbA1c of 7% and above at baseline
In a prespecified subgroup of patients with baseline HbA1c greater or equal than 8.5% the reduction from baseline in HbA1c with empagliflozin 25 mg/linagliptin 5 mg was -1.8% at 24 weeks (p<0.0001 versus linagliptin 5 mg, p<0.001 versus empagliflozin 25 mg) and -1.8% at 52 weeks (p<0.0001 versus linagliptin 5 mg, p<0.05 versus empagliflozin 25 mg) and with empagliflozin 10 mg/5 mg linagliptin -1.6% at 24 weeks (p<0.01 versus linagliptin 5 mg, n.s. versus empagliflozin 10 mg) and -1.5% at 52 weeks (p<0.01 versus linagliptin 5 mg, n.s. versus empagliflozin 10 mg).
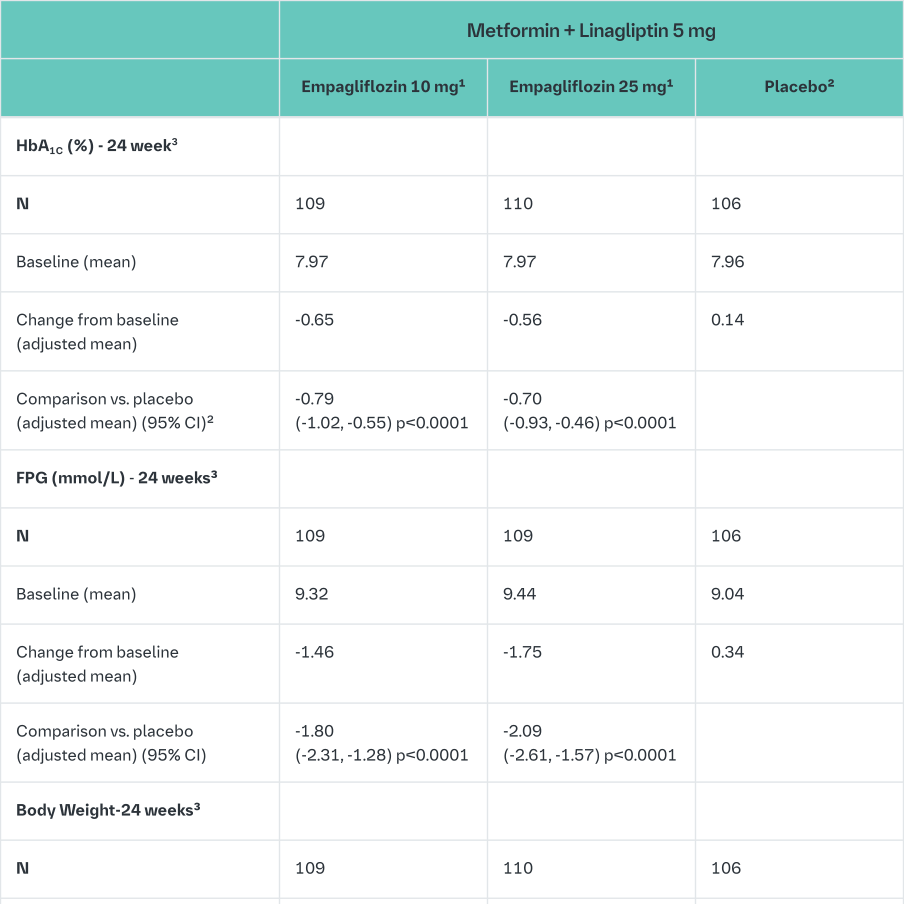
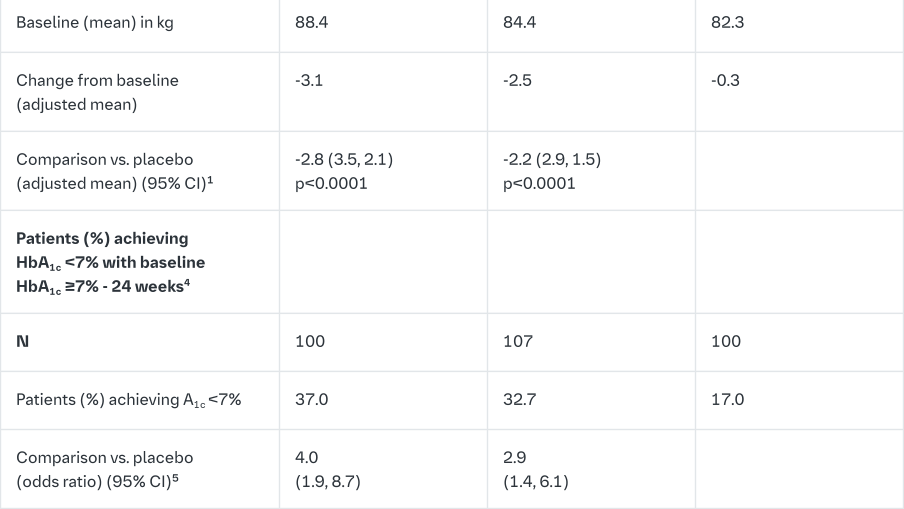
Empagliflozin vs. placebo in patients inadequately controlled on metformin and linagliptin
In patients inadequately controlled on metformin and linagliptin, 24-weeks treatment with both doses (10 mg and 25 mg) of empagliflozin provided statistically significant improvements in HbA1c, FPG and body weight compared to placebo (background linagliptin 5 mg). A statistically significant greater number of patients with a baseline HbA1c ≥7.0% and treated with empagliflozin achieved a target HbA1c of <7% compared to placebo (background linagliptin 5 mg) (Table 11). After 24 weeks’ treatment with empagliflozin, both SBPs and DBPs were reduced, -2.6/-1.1 mmHg (n.s. versus placebo for SBP and DBP) for empagliflozin 25 mg/linagliptin 5 mg and -1.3/-0.1 mmHg (n.s. versus placebo for SBP and DBP) for empagliflozin 10 mg/linagliptin 5 mg.
After 24 weeks, rescue therapy was used in 4 (3.6%) patients treated with empagliflozin 25 mg/linagliptin 5 mg and in 2 (1.8%) patients treated with empagliflozin 10 mg/linagliptin 5 mg, compared to 13 (12.0%) patients treated with placebo (background linagliptin 5 mg).
1Patients randomized to the empagliflozin 10 mg group were receiving empagliflozin 10 mg/linagliptin 5 mg or empagliflozin 25 mg/linagliptin 5 mg with background metformin
2Patients randomized to the placebo group were receiving the placebo plus linagliptin 5 mg with background metformin
3MMRM model on FAS (OC) includes baseline HbA1c baseline eGFR (MDRD), geographical region, visit treatment, and visit by treatment interaction. For FPG, baseline FPG is also included. For weight, baseline weight is also included
4not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
5Logistic regression on FAS (NCF) includes baseline HbA1c, baseline eGFR (MDRD), geographical region, and treatment; based on patients with HbA1c of 7% and above at baseline
In a prespecified subgroup of patients with baseline HbA1c greater or equal than 8.5% the reduction from baseline in HbA1c with empagliflozin 25 mg/linagliptin 5 mg was -1.3% at 24 weeks (p<0.0001 versus placebo [background linagliptin 5 mg]) and with empagliflozin 10 mg/linagliptin 5 mg -1.3% at 24 weeks (p<0.0001 versus placebo [background linagliptin 5 mg]).
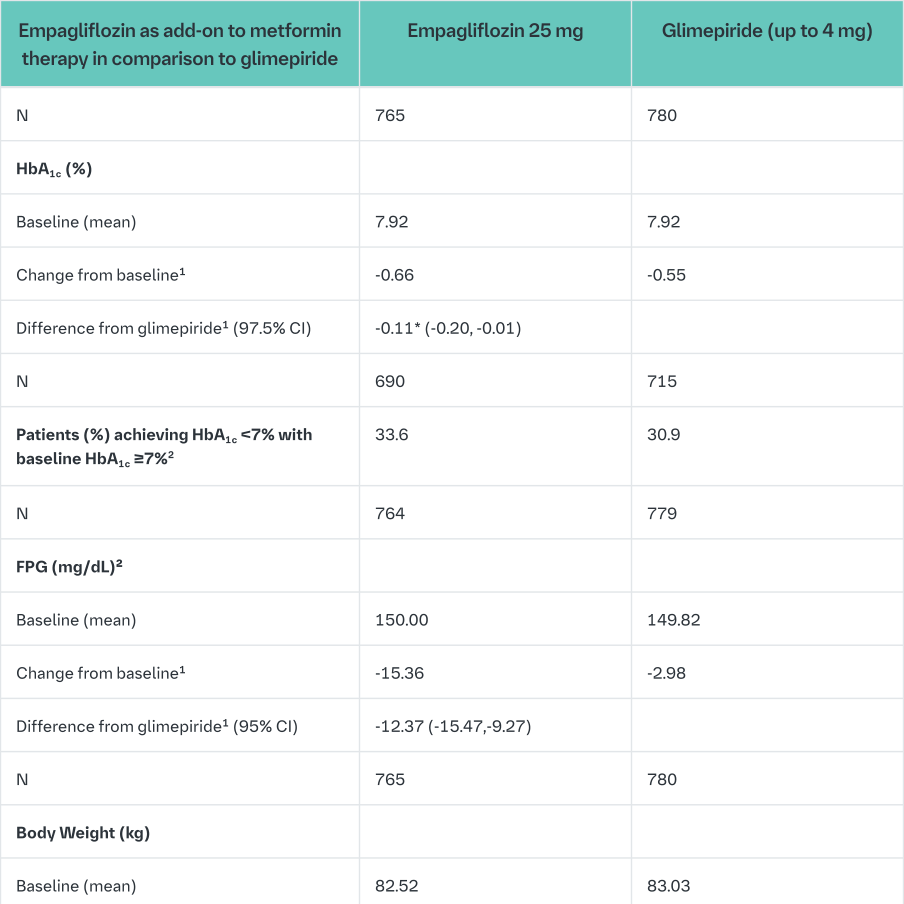
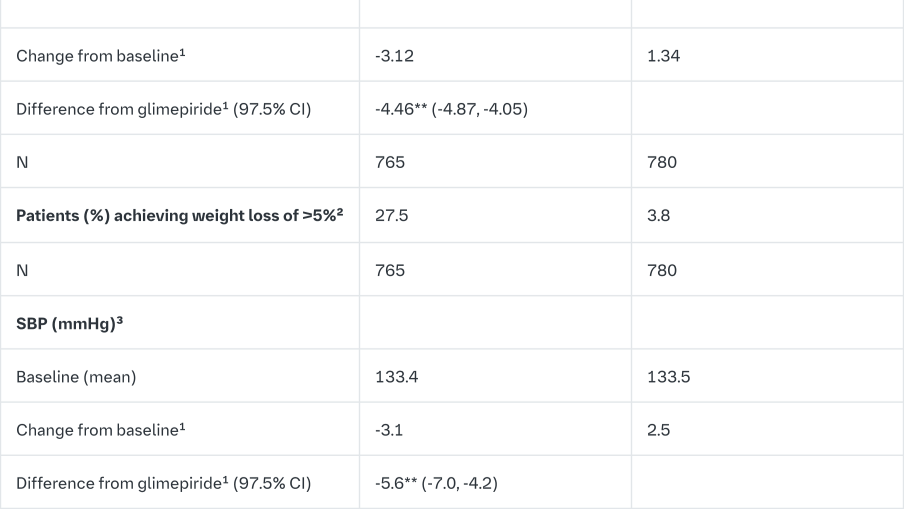
Empagliflozin 2-year data, as add on to metformin in comparison to glimepiride
In a study comparing the efficacy and safety of empagliflozin 25 mg versus glimepiride (4 mg) in patients with inadequate glycaemic control on metformin alone, treatment with empagliflozin 25 mg daily resulted in superior reduction in HbA1c, and a clinically meaningful reduction in FPG, compared to glimepiride (Table 12). Empagliflozin 25 mg daily resulted in a statistically significant reduction in body weight, systolic and diastolic blood pressure (change from baseline in DBP of -1.8 mmHg for empagliflozin and +0.9 mmHg for glimepiride, p<0.0001).
Treatment with empagliflozin 25 mg daily resulted in statistically significantly lower proportion of patients with hypoglycaemic events compared to glimepiride (2.5% for empagliflozin 25 mg, 24.2% for glimepiride, p<0.0001).
1mean adjusted for baseline value and stratification
2not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
3Last observation (prior glycemic rescue or to antihypertensive rescue) carried forward (LOCF)
4Last observation (prior to glycemic rescue) carried forward (LOCF)
*p-value <0.0001 for non-inferiority, and p-value = 0.0153 for superiority
**p-value <0.0001
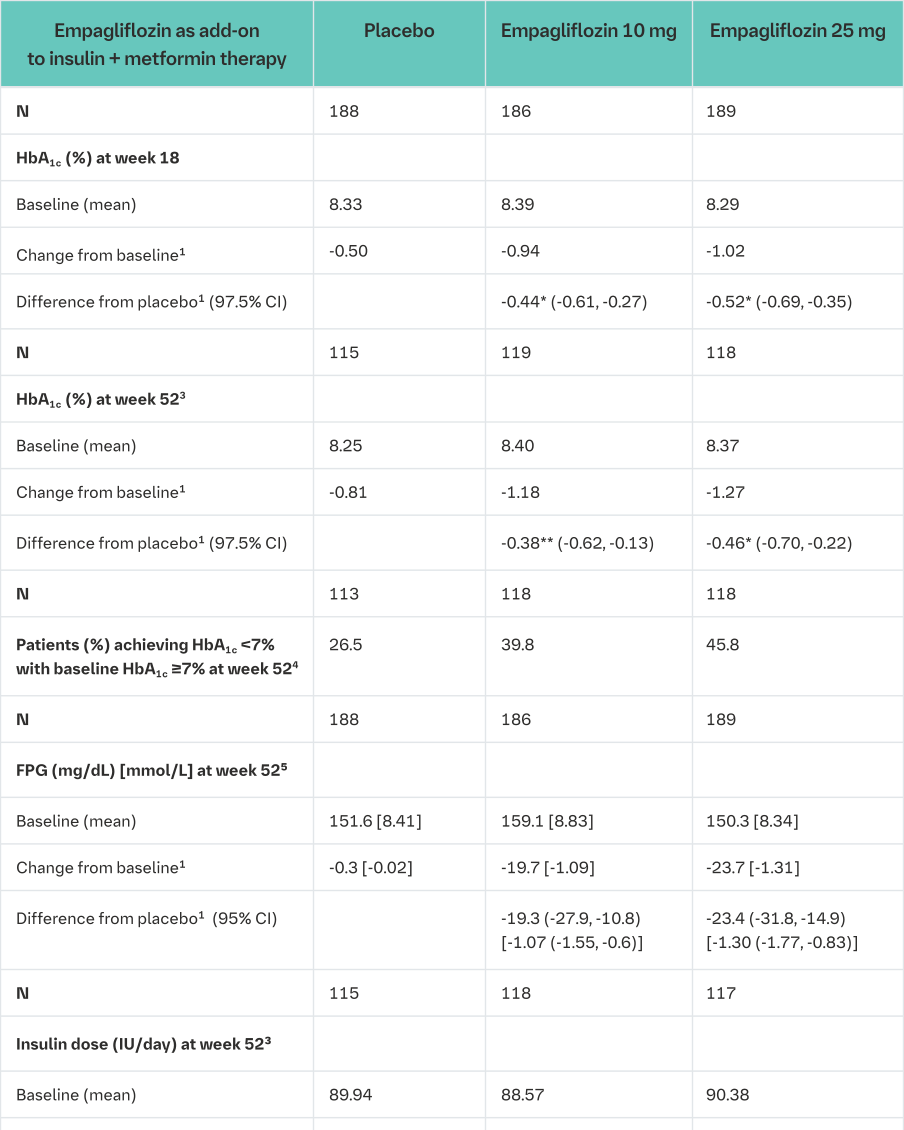
Empagliflozin as add on to MDI insulin therapy and metformin
The efficacy and safety of empagliflozin as add-on to multiple daily insulin with or without concomitant metformin therapy (71.0% of all patients were on metformin background) was evaluated in a double-blind, placebo-controlled trial of 52 weeks duration. During the initial 18 weeks and the last 12 weeks, the insulin dose was to be kept stable, but was adjusted to achieve pre-prandial glucose levels <100 mg/dL [5.5 mmol/L], and post- prandial glucose levels <140 mg/dL [7.8 mmol/L] between Weeks 19 and 40.
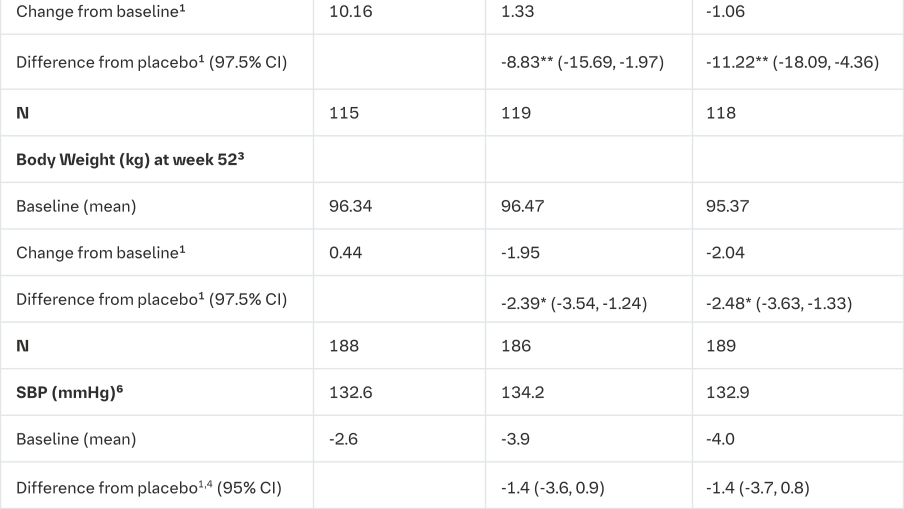
At Week 18, empagliflozin provided statistically significant improvement in HbA1c compared with placebo (Table 13). A greater proportion of patients with a baseline HbA1c ≥7.0% (19.5% empagliflozin 10 mg, 31.0% empagliflozin 25 mg) achieved a target HbA1c of <7% compared with placebo (15.1%).
At Week 52, treatment with empagliflozin resulted in a statistically significant decrease in HbA1c and insulin sparing compared with placebo and a reduction in FPG (change from baseline of -0.3 mg/dL [-0.02 mmol/L] for placebo, -19.7 mg/dL [-1.09 mmol/L] for empagliflozin 10 mg, and -23.7 mg/dL [-1.31 mmol/L] for empagliflozin 25 mg), body weight, and blood pressure (SBP: change from baseline of -2.6 mmHg for placebo, -3.9 mmHg for empagliflozin 10 mg and -4.0 mmHg for empagliflozin 25 mg, DBP: change from baseline of -1.0 mmHg for placebo, -1.4 mmHg for empagliflozin 10 mg and -2.6 mmHg for empagliflozin 25 mg).
1mean adjusted for baseline value and stratification
2Week 18: FAS; week 52: PPS-Completers-52
3Week 19-40: treat-to-target regimen for insulin dose adjustment to achieve pre-defined glucose target levels (pre-prandial <100 mg/dL (5.5 mmol/L), post-prandial <140 mg/dL (7.8 mmol/L)
4not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
5Last observation (prior to glycaemic rescue) carried forward (LOCF)
6Week 52: FAS
*p-value <0.0001
**p-value <0.005
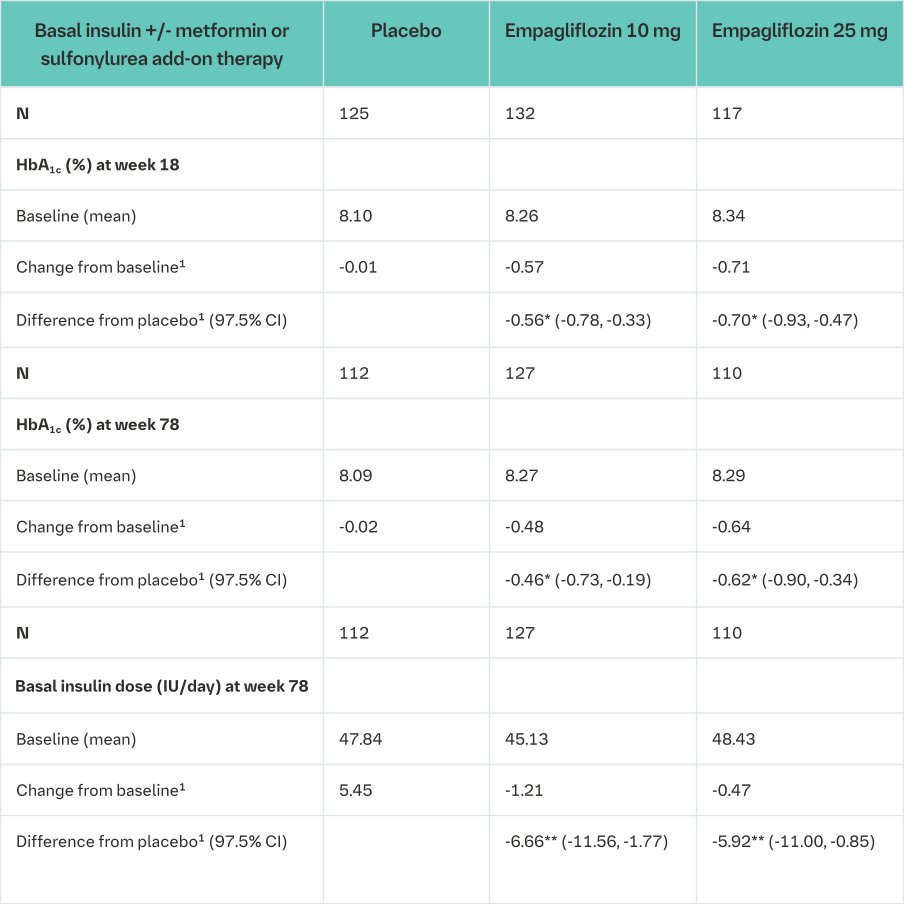
Empagliflozin as add on to basal insulin therapy
The efficacy and safety of empagliflozin (10 mg or 25 mg) as add on to basal insulin with or without concomitant metformin and/or sulfonylurea therapy was evaluated in a double-blind, placebo-controlled trial of 78 weeks duration. During the initial 18 weeks, the insulin dose was to be kept stable, but was adjusted to achieve a FPG <110 mg/dL in the following 60 weeks.
At week 18, empagliflozin (10 mg or 25 mg) provided statistically significant improvement in HbA1c compared to placebo. A greater proportion of patients with a baseline HbA1c ≥7.0% achieved a target HbA1c of <7% compared to placebo. At 78 weeks, empagliflozin resulted in a statistically significant decrease in HbA1c and insulin sparing compared to placebo (Table 14).
At week 78, empagliflozin resulted in a reduction in FPG -10.51 mg/dL [-0.58 mmol/L] for empagliflozin 10 mg, -17.43 mg/dL [-0.3 mmol/L] for empagliflozin 25 mg and -5.48 mg/dL [- 0.97 mmol/L] for placebo), body weight (-2.47 kg for empagliflozin 10 mg, -1.96 kg for empagliflozin 25 mg and +1.16 kg for placebo, p<0.0001), blood pressure (SBP: -4.1 mmHg for empagliflozin 10 mg, -2.4 mmHg for empagliflozin 25 mg and +0.1 mmHg for placebo, DPB: -2.9 mmHg for empagliflozin 10 mg, -1.5 mmHg for empagliflozin 25 mg and -0.3 mmHg for placebo).
1mean adjusted for baseline value and stratification
*p-value <0.0001
**p-value <0.025
Empagliflozin as add on to dipeptidyl peptidase 4 (DPP-4) inhibitor therapy
The efficacy and safety of empagliflozin as add on to DPP-4 inhibitors plus metformin, with or without one additional oral antidiabetes drug was evaluated in 160 patients with high cardiovascular risk. Treatment with empagliflozin for 28 weeks reduced HbA1c compared to placebo (change from baseline -0.54% for empagliflozin 10 mg, -0.52% for empagliflozin 25 mg, and -0.02% for placebo).
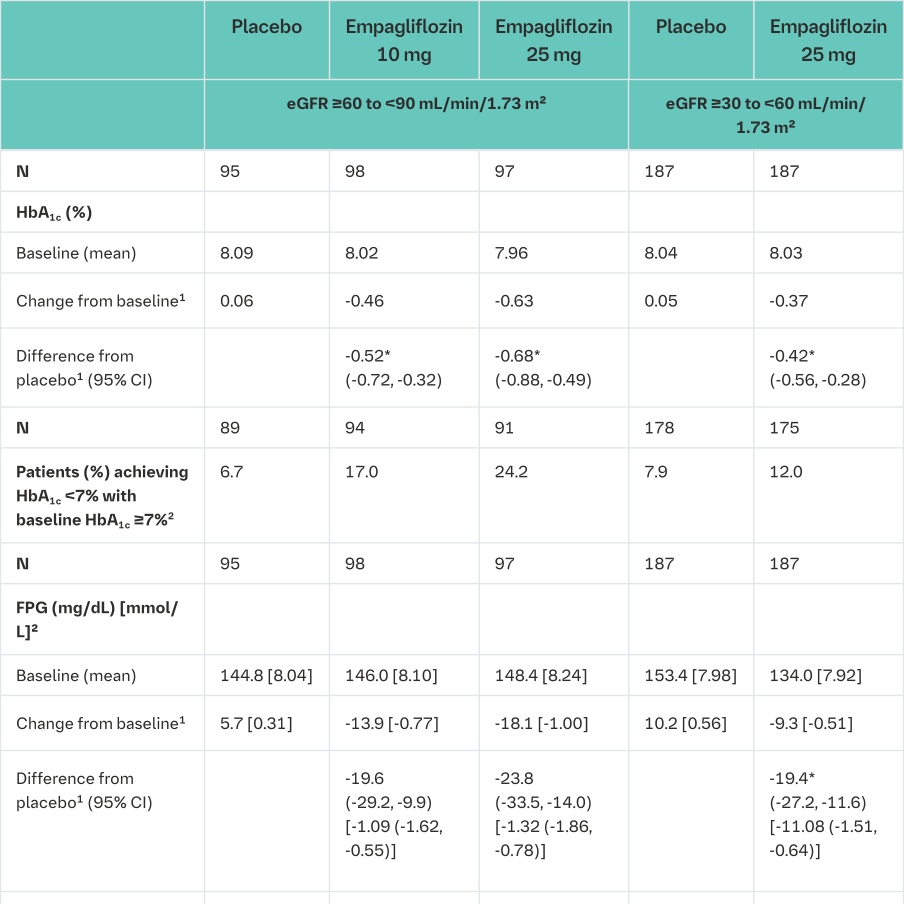
Patients with renal impairment, 52-week placebo-controlled data
The efficacy and safety of empagliflozin as add on to antidiabetic therapy was evaluated in patients with mild and moderate renal impairment in a double-blind, placebo-controlled study for 52 weeks.
Treatment with Empagliflozin (Jardiance) led to statistically significant reduction of HbA1c and clinically meaningful improvement in FPG (fasting plasma glucose), body weight and blood pressure compared to placebo at Week 24 (Table 15). The improvement in HbA1c, FPG, body weight, and blood pressure was sustained up to 52 weeks.
1mean adjusted for baseline value and stratification
2not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
*p<0.0001
2 hour post-prandial glucose
Treatment with empagliflozin (10 mg or 25 mg) as add-on to metformin or metformin plus sulfonylurea resulted in clinically meaningful improvement of 2-hour post-prandial glucose (meal tolerance test) at 24 weeks (add-on to metformin, placebo (n=57) +5.9 mg/dL, empagliflozin 10 mg (n=52): -46.0 mg/dL, empagliflozin 25 mg (n=58): -44.6 mg/dL; add- on to metformin plus sulphonylurea, placebo (n=35): -2.3 mg/dL, empagliflozin 10 mg (n=44): -35.7 mg/dL, empagliflozin 25 mg (n=46): -36.6 mg/dL).
Patients with high baseline HbA1c >10%
In a pre-specified pooled analysis of three phase 3 studies, treatment with open-label empagliflozin 25 mg in patients with severe hyperglycaemia (N=184 mean baseline HbA1c 11.15%) resulted in a clinically meaningful reduction in HbA1c from baseline (- 3.27%) at week 24.
Body weight
In a pre-specified pooled analysis of 4 placebo-controlled studies, treatment with empagliflozin resulted in body weight reduction compared to placebo at week 24 (-2.04 kg for empagliflozin 10 mg, -2.26 kg for empagliflozin 25 mg and -0.24 kg for placebo) that was maintained up to week 52 (-1.96 kg for empagliflozin 10 mg, -2.25 kg for empagliflozin 25 mg and -0.16 kg for placebo).
Waist circumference
Treatment with empagliflozin as monotherapy or as add-on to metformin, pioglitazone, or metformin plus sulphonylurea resulted in sustained reduction of waist circumference over the duration of studies in a range of -1.7 cm to -0.9 cm for empagliflozin and -0.5 cm to +0.2 cm for placebo.
Blood pressure
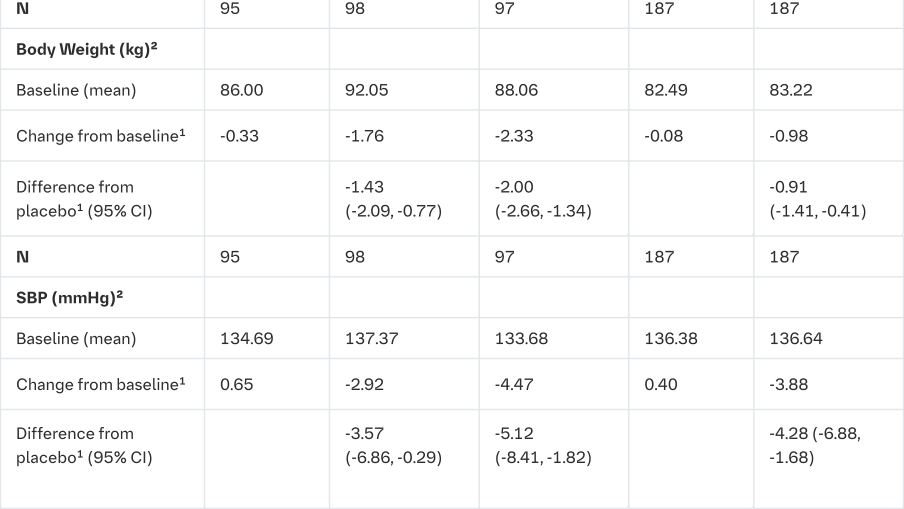
The efficacy and safety of empagliflozin (10 mg or 25 mg) was evaluated in a double-blind, placebo-controlled study of 12 weeks duration in patients with type 2 diabetes and high blood pressure on different antidiabetic and up to 2 antihypertensive therapies (Table 16).
Treatment with empagliflozin once daily resulted in statistically significant improvement in HbA1c, 24 hour mean systolic and diastolic blood pressure as determined by ambulatory blood pressure monitoring. Treatment with empagliflozin provided reductions in seated SBP (change from baseline of -0.67 mmHg for placebo, -4.60 mmHg for empagliflozin 10 mg and -5.47 mmHg for empagliflozin 25 mg) and seated DBP (change from baseline of -1.13 mmHg for placebo, -3.06 mmHg for empagliflozin 10 mg and -3.02 mmHg for empagliflozin 25 mg).
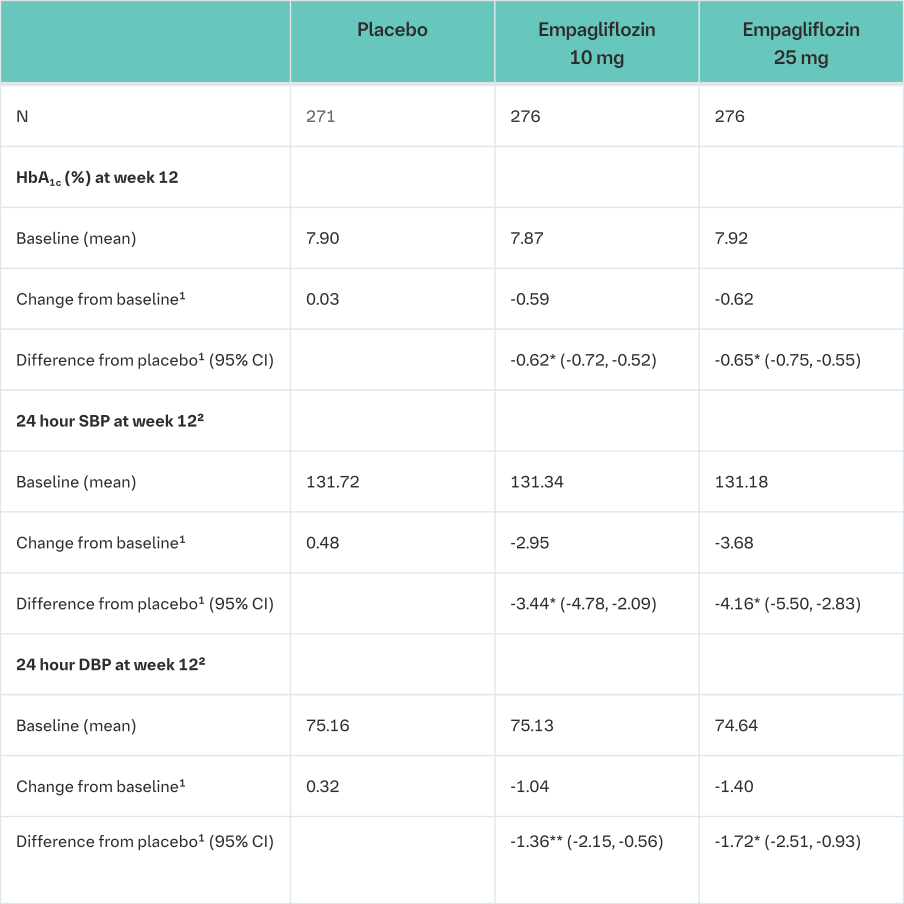
1mean adjusted for baseline value and stratification
2Last observation (prior to antihypertensive rescue) carried forward (LOCF)
3Last observation (prior to glycaemic rescue) carried forward (LOCF)
*p-value <0.0001
**p-value =0.0008
In a pre-specified pooled analysis of 4 placebo-controlled studies, treatment with empagliflozin resulted in a reduction in systolic blood pressure (empagliflozin 10 mg -3.9 mmHg, empagliflozin 25 mg -4.3 mmHg) compared with placebo (-0.5 mmHg), and in diastolic blood pressure (empagliflozin 10 mg -1.8 mmHg, empagliflozin 25 mg -2.0 mmHg) compared with placebo (-0.5 mmHg), at week 24, that were maintained up to week 52.
Laboratory parameters
Haematocrit increased
In a pooled safety analysis (pooling of all patients with diabetes, n=13,402), mean changes from baseline in haematocrit were 3.4% and 3.6% for empagliflozin 10 mg and 25 mg, respectively, compared to -0.1% for placebo. In the EMPA-REG Outcome study, haematocrit values returned towards baseline values after a follow-up period of 30 days after treatment stop.
Serum lipids increased
In a pooled safety analysis (pooling of all patients with diabetes, n=13,402), mean percent increases from baseline for empagliflozin 10 mg and 25 mg versus placebo, respectively, were total cholesterol 4.9% and 5.7% versus 3.5%; HDL-cholesterol 3.3% and 3.6% versus 0.4%; LDL-cholesterol 9.5% and 10.0% versus 7.5%; triglycerides 9.2% and 9.9% versus 10.5%.
Cardiovascular outcome
The EMPA-REG OUTCOME® study is a multi-centre, multi-national, randomized, double-blind, placebo-controlled trial investigating the effect of Empagliflozin (Jardiance) as adjunct to standard care therapy in reducing cardiovascular events in patients with type 2 diabetes and one or more cardiovascular risk factors, including coronary artery disease, peripheral artery disease, history of myocardial infarction (MI), or history of stroke. The primary endpoint was the time to first event in the composite of CV death, nonfatal MI, or non-fatal stroke (Major Adverse Cardiovascular Events (MACE-3)). Additional pre-specified endpoints addressing clinically relevant outcomes tested in an exploratory manner included CV death, the composite of heart failure requiring hospitalisation or CV death, all-cause mortality and the composite of new or worsening nephropathy.
A total of 7020 patients were treated with Empagliflozin (Jardiance) (empagliflozin 10 mg: 2345, empagliflozin 25 mg: 2342, placebo: 2333) and followed for a median of 3.1 years. The population was 72.4% Caucasian, 21.6% Asian, and 5.1% Black. The mean age was 63 years and 71.5% were male. At baseline, approximately 81% of patients were being treated with renin angiotensin system inhibitors, 65% with beta-blockers, 43% with diuretics, 89% with anticoagulants, and 81% with lipid lowering medication. Approximately 74% of patients were being treated with metformin at baseline, 48% with insulin and 43% with sulphonylurea.
About half of the patients (52.2%) had an eGFR of 60-90 mL/min/1.73 m², 17.8% of 45-60 mL/min/1.73 m² and 7.7% of 30-45 mL/min/1.73 m². Mean systolic BP was 136 mmHg, diastolic BP 76 mmHg, LDL 86 mg/dL, HDL 44 mg/dL, and urinary albumin to creatinine ratio (UACR) 175 mg/g at baseline.
Reductions in risk of CV death and all-cause mortality
Empagliflozin (Jardiance) was superior in reducing the primary composite endpoint of cardiovascular death, non-fatal MI, or non-fatal stroke compared to placebo. The treatment effect reflected a significant reduction in cardiovascular death with no significant change in non-fatal MI, or non-fatal stroke (Table 17 and Figure 1).
Empagliflozin (Jardiance) also improved overall survival (Table 17 and Figure 2), which was driven by a reduction in cardiovascular death with Empagliflozin (Jardiance). There was no statistically significant difference between empagliflozin and placebo in non-cardiovascular mortality.
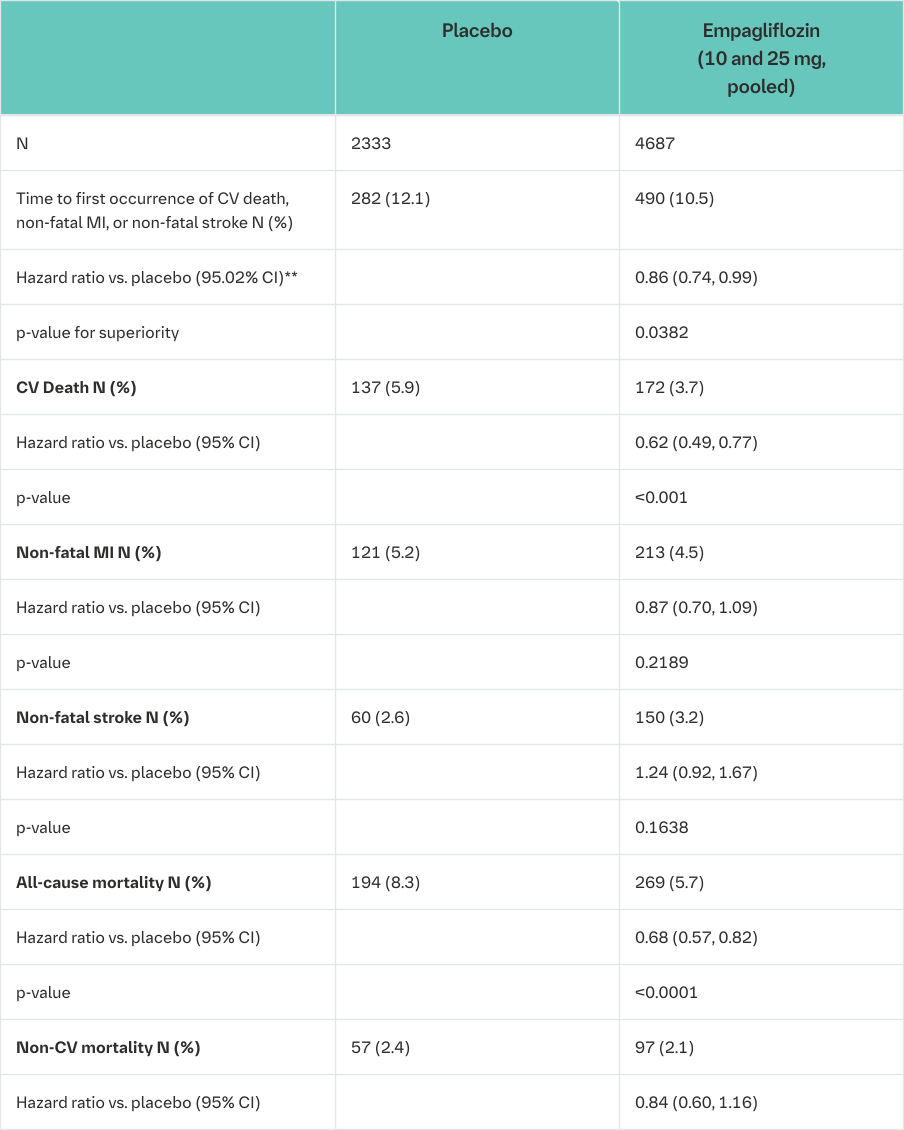
*i.e. patients who had received at least one dose of study drug
**Since data from the trial were included in an interim analysis, a two-sided 95.02% confidence interval applied which corresponds to a p-value of less than 0.0498 for significance
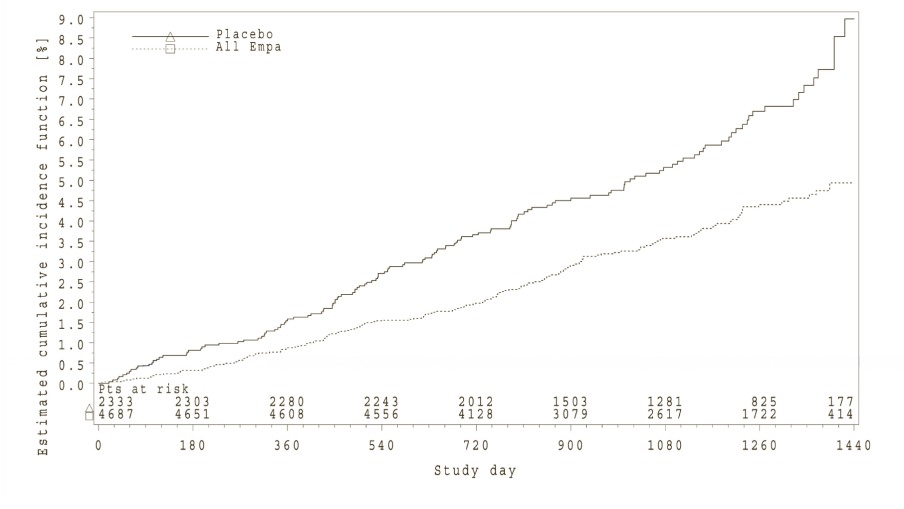
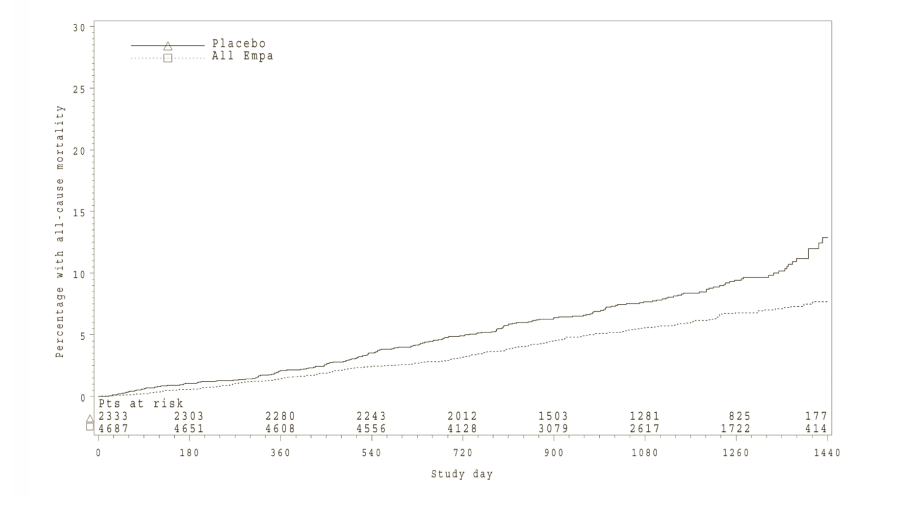
*Kaplan-Meier estimate of time to all cause-mortality, pooled empagliflozin vs. placebo-treated set
Reductions in risk of heart failure requiring hospitalisation or CV death
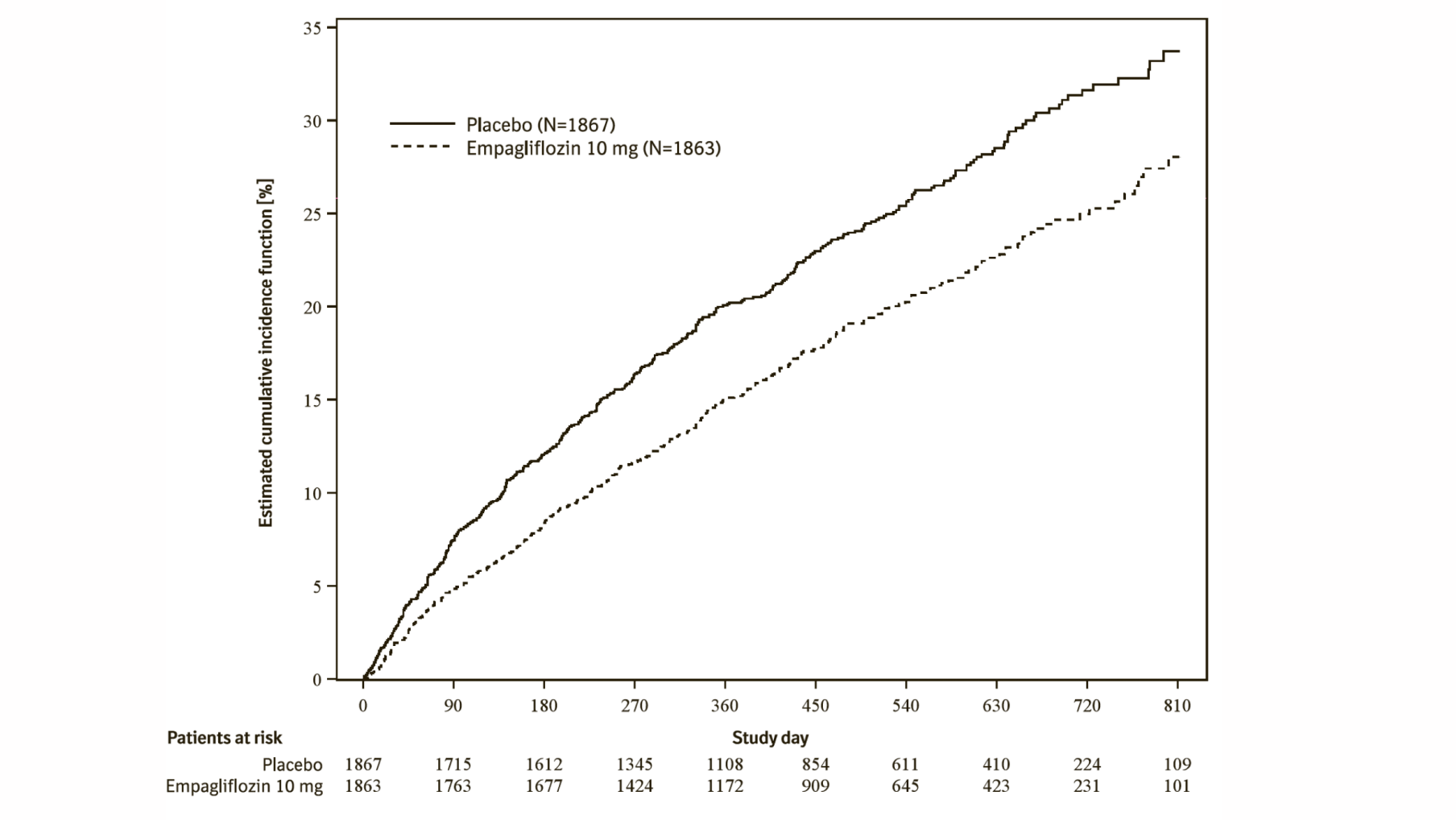
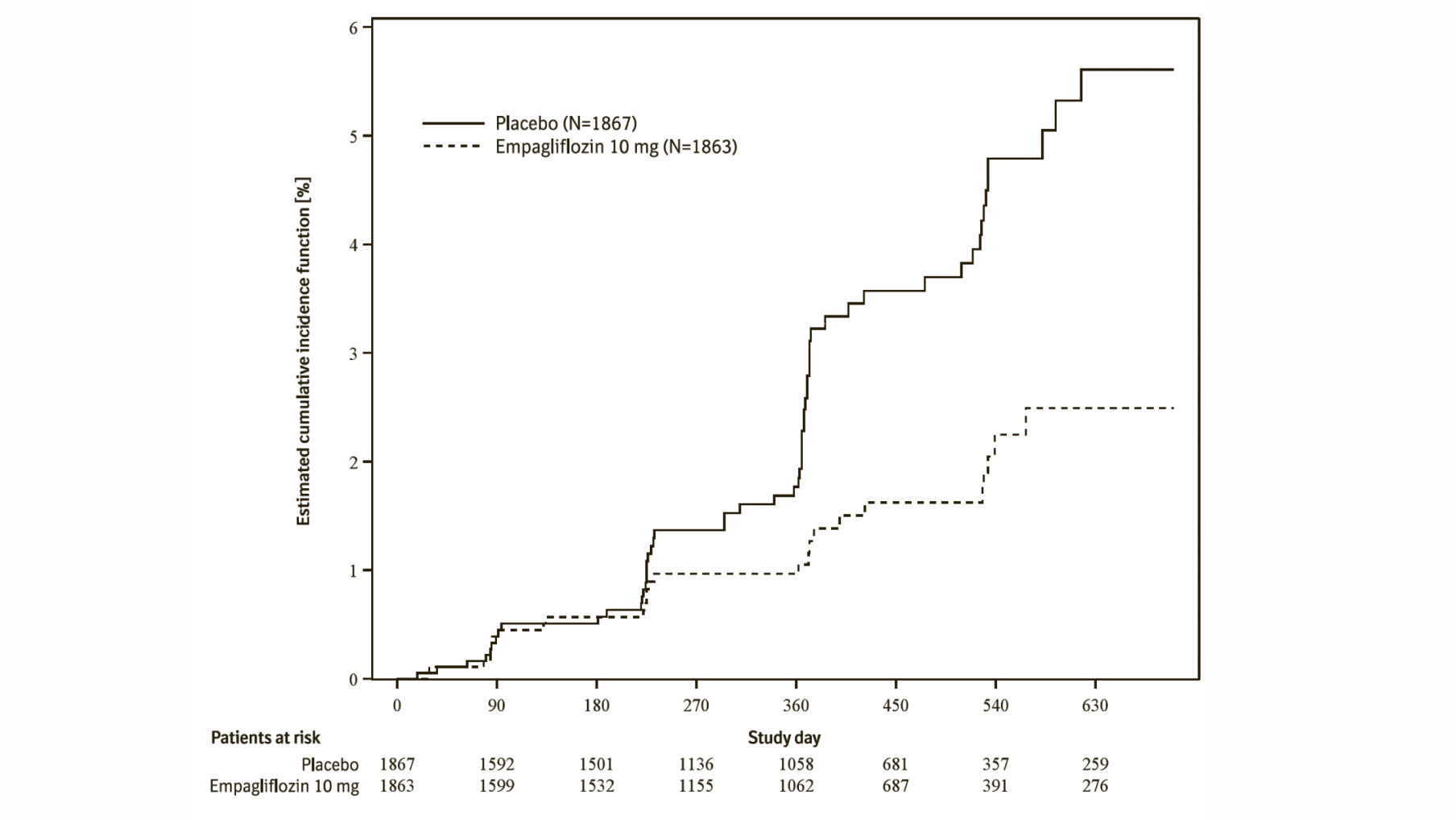
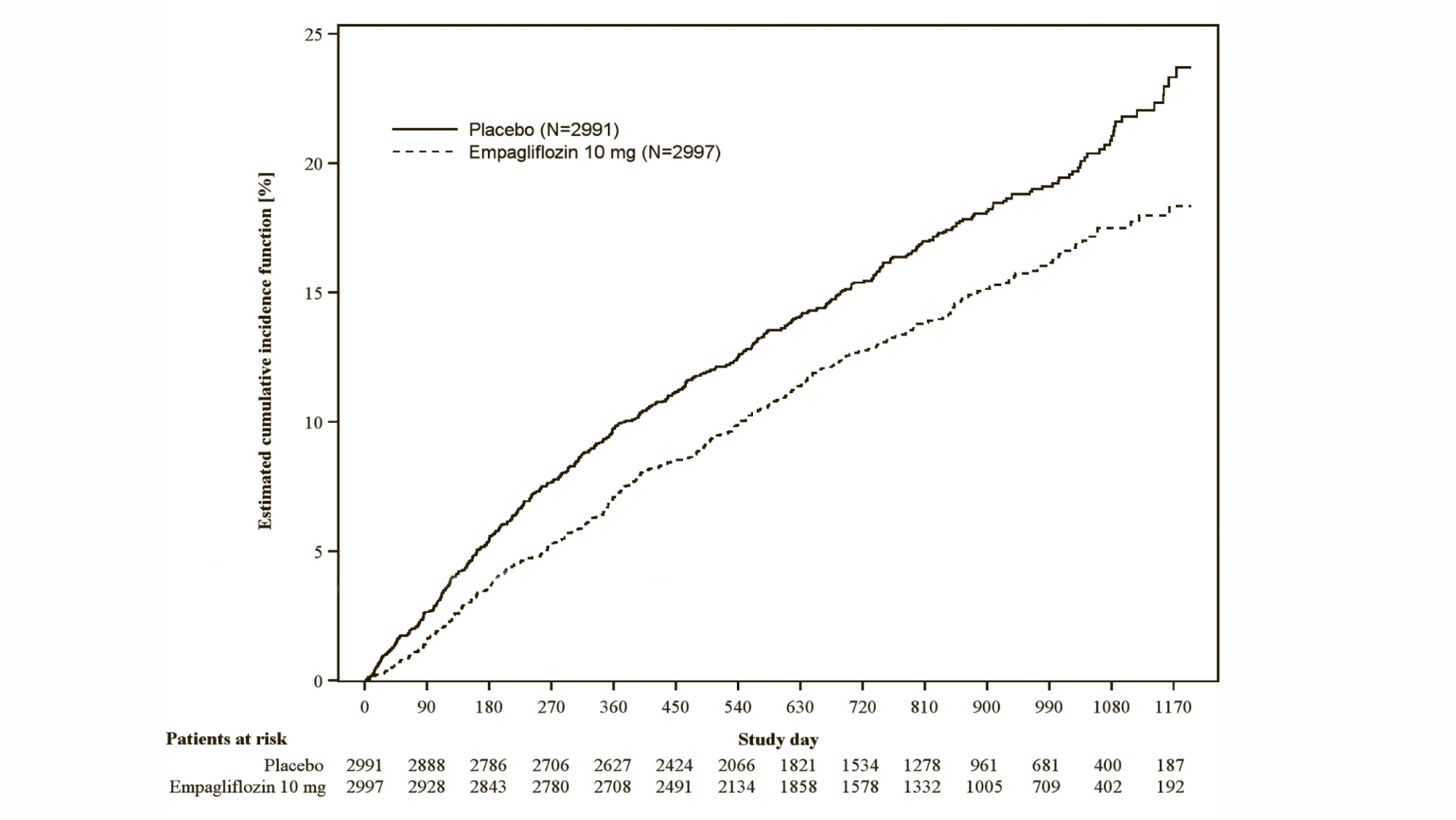
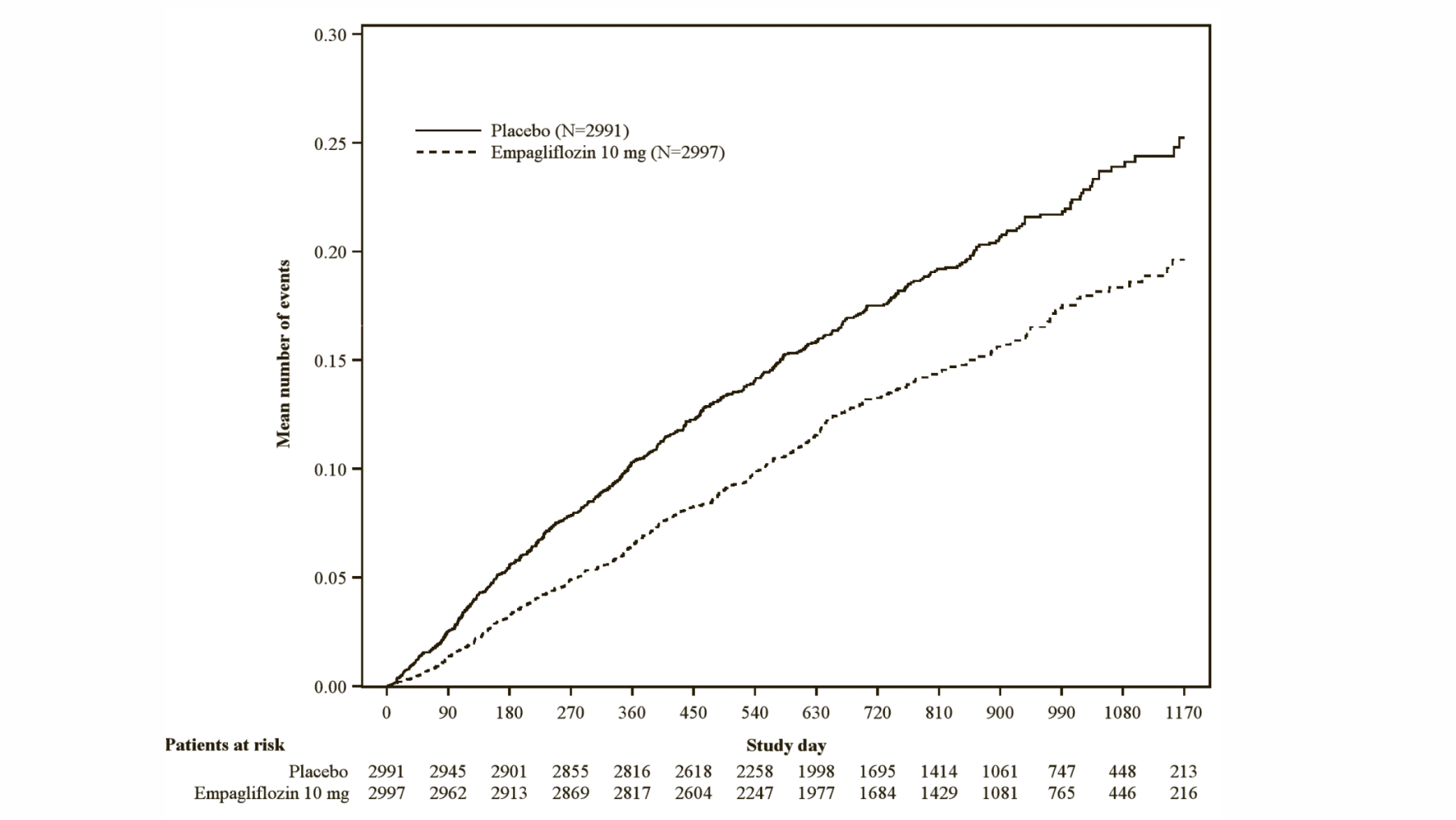
Empagliflozin (Jardiance) significantly reduced the risk of hospitalisation for heart failure and cardiovascular death or hospitalisation for heart failure compared with placebo (Table 18 and Figure 3).
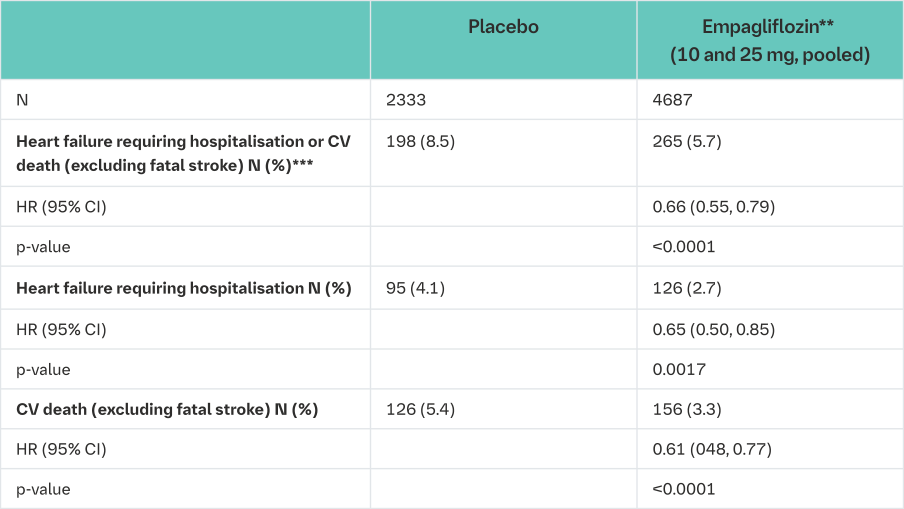
*i.e. patients who had received at least one dose of study drug
**empagliflozin 10 mg and 25 mg showed consistent results
***time to first event
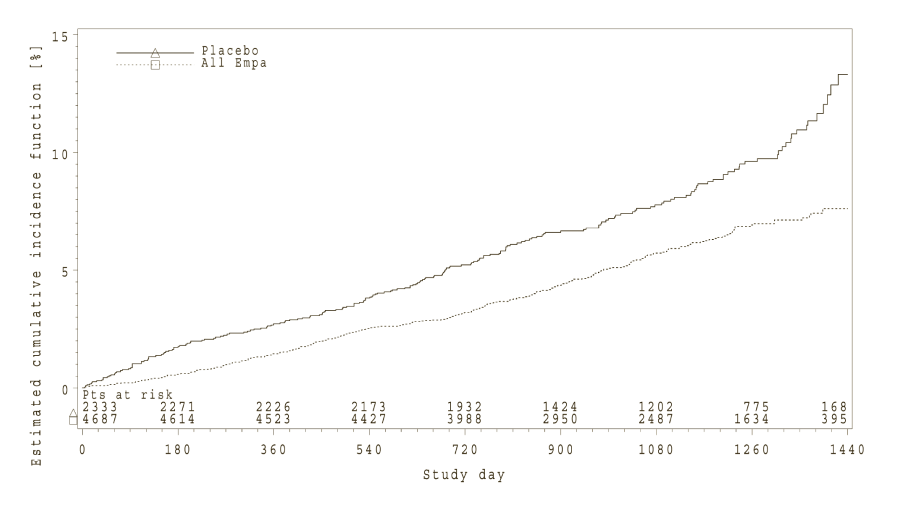
*Estimated cumulative incidence function for time to first occurrence of first heart failure hospitalisation or CV death, pooled empagliflozin vs placebo – treated set
The cardiovascular benefits of Empagliflozin (Jardiance) observed were consistent across the subgroups depicted in Figure 4.
EMPA-REG OUTCOME subgroup analyses
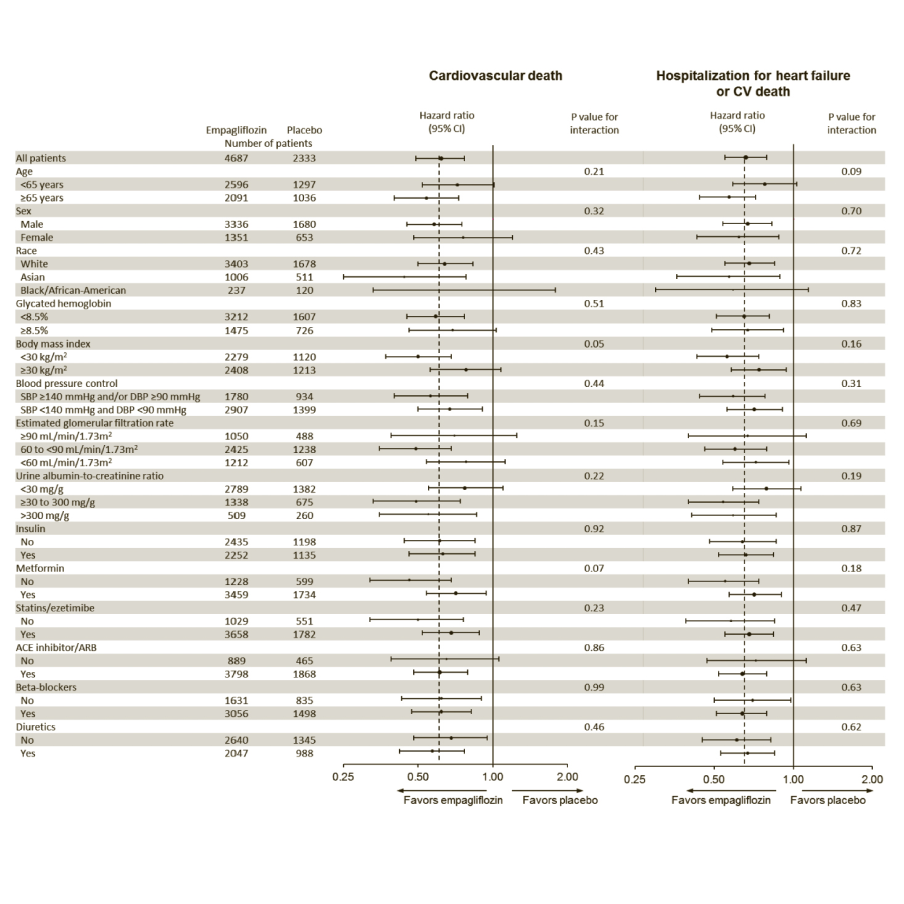
Cox regression analysis in patients treated with ≥1 dose of study drug.
p-value is for test of homogeneity of treatment group difference among subgroups (test for group by covariate interaction) with no adjustment for multiple tests.
*Hospitalisation for heart failure or CV death excludes fatal stroke
**p-value is for test of homogeneity of treatment group difference among subgroups (test for group by covariate interaction) with no adjustment for multiple tests and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted
Diabetic kidney disease
In the EMPA-REG OUTCOME® study population, the risk of new or worsening nephropathy (defined as onset of macroalbuminuria, doubling of serum creatinine, and initiation of renal replacement therapy (i.e. hemodialysis)) was significantly reduced in empagliflozin group compared to placebo (Table 19 and Figure 5).
Empagliflozin (Jardiance) compared with placebo showed a significantly higher occurrence of sustained normo- or microalbuminuria in patients with baseline macroalbuminuria (HR 1.82, 95% CI 1.40, 2.37).
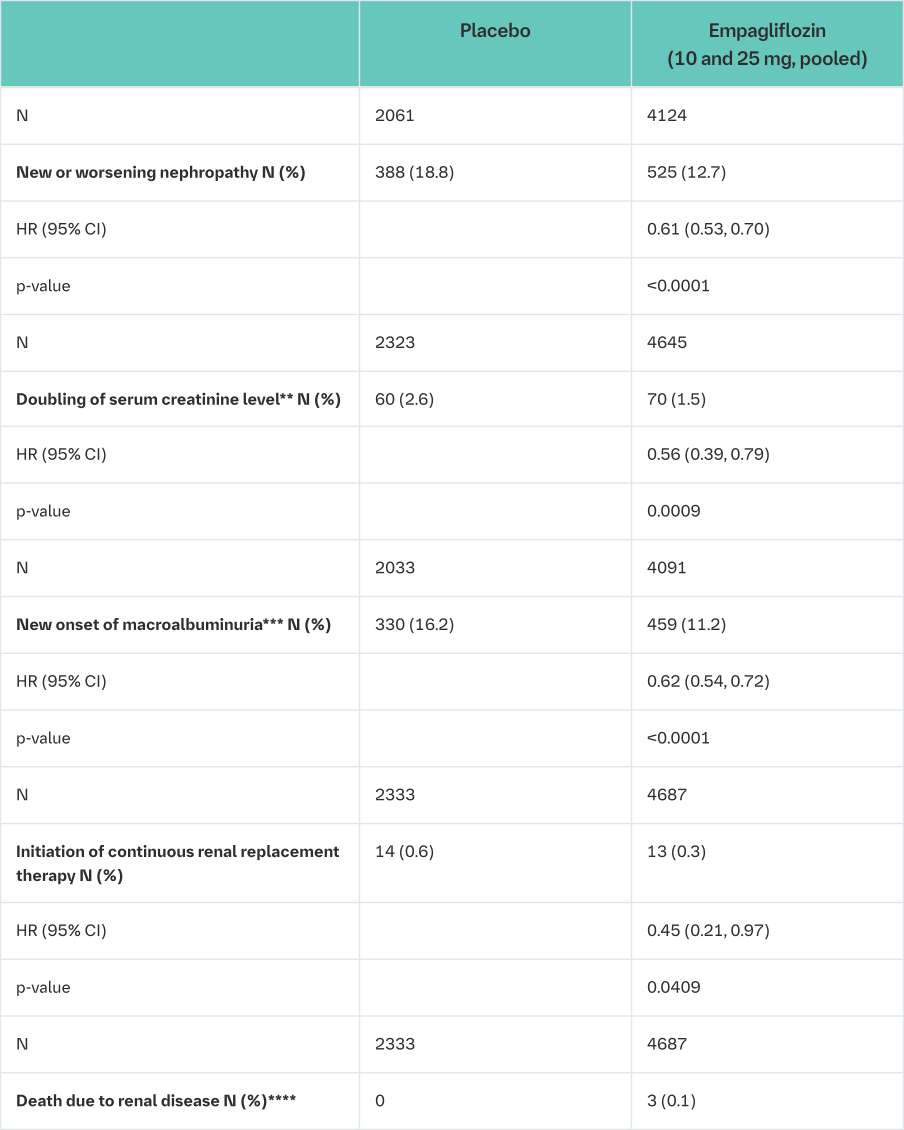
*i.e. patients who had received at least one dose of study drug
**Accompanied by an eGFR ≤45 mL/min/1.73 m2
***Urine Albumin Creatinine Ratio >300 mg/g
****Due to low event rate, HR not calculated
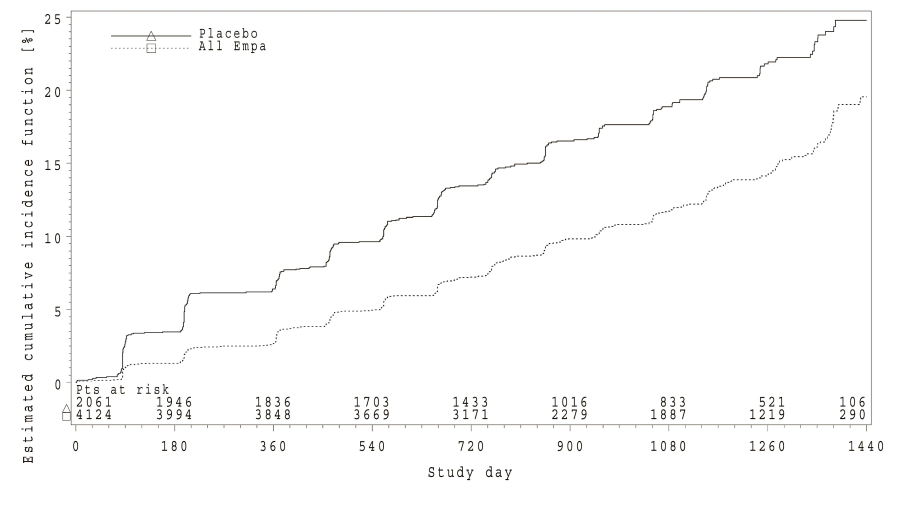
Treatment with empagliflozin preserved eGFR and eGFR increased during the post treatment 4-week follow up. However, the placebo group showed a gradual decline in GFR during the course of the study with no further change during 4-week follow up. (see Figure 6).
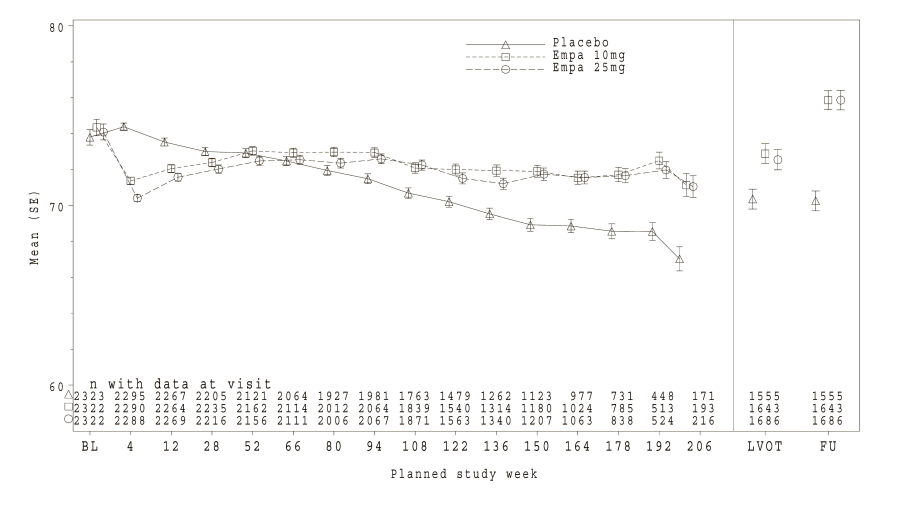
*eGFR (MDRD) (mL/min/1.73 m²) MMRM results over time, unadjusted last value on treatment and follow-up value - treated set – right side based on patients with available last value on treatment (LVOT) and follow-up (FU)
Thorough QTc study
In a randomised, placebo-controlled, active-comparator, crossover study of 30 healthy subjects, no increase in QTc was observed with either 25 mg or 200 mg empagliflozin.
Heart failure
Empagliflozin in patients with heart failure and reduced ejection fraction.
A randomized, double-blind, placebo-controlled study (EMPEROR-Reduced) was conducted in 3730 patients with chronic heart failure (New York Heart Association [NYHA] II-IV) and reduced ejection fraction (LVEF ≤40%) to evaluate the efficacy and safety of empagliflozin 10 mg once daily as adjunct to standard of care heart failure therapy. The primary endpoint was the time to adjudicated first event of either cardiovascular (CV) death or hospitalisation for heart failure (HHF). Occurrence of adjudicated HHF (first and recurrent), and eGFR (CKD-EPI)cr slope of change from baseline were included in the confirmatory testing. Heart Failure therapy at baseline included ACE inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitor (88.3%), beta blockers (94.7%), mineralocorticoid receptor antagonists (71.3%) and diuretics (95.0%).
A total of 1863 patients were randomized to empagliflozin 10 mg (placebo: 1867) and followed for a median of 15.7 months. The study population consisted of 76.1% men and 23.9% women with a mean age of 66.8 years (range: 25-94 years), 26.8% were 75 years of age or older. 70.5% of the study population were White, 18.0% Asian and 6.9% Black/African American. At randomization, 75.1% of patients were NYHA class II, 24.4% were class III and 0.5% were class IV. The mean LVEF was 27.5%. At baseline, the mean eGFR was 62.0 mL/min/1.73 m² and the median urinary albumin to creatinine ratio (UACR) was 22 mg/g. About half of the patients (51.7%) had an eGFR of ≥60 mL/min/1.73 m², 24.1% of 45 to <60 mL/min/1.73 m², 18.6% of 30 to <45 mL/min/1.73 m² and 5.3% 20 to <30 mL/min/1.73 m².
Empagliflozin was superior in reducing the risk of the primary composite endpoint of cardiovascular death or hospitalisation for heart failure compared with placebo.
Additionally, empagliflozin significantly reduced the risk of occurrence of HHF (first and recurrent), and significantly reduced the rate of eGFR decline. (see Table 20).
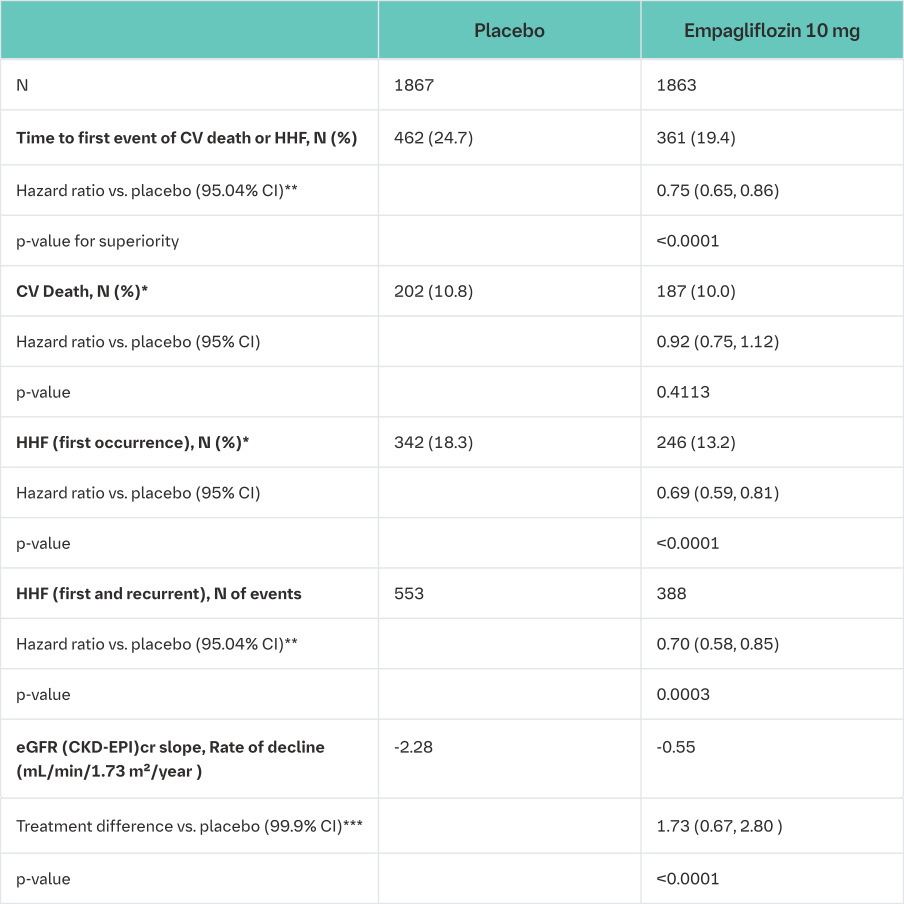
CV = cardiovascular, HHF = hospitalisation for heart failure, eGFR = Estimated glomerular filtration rate, CKD EPI = Chronic kidney disease epidemiology collaboration equation
*not controlled for type 1 error
**Due to an interim analysis, a two-sided 95.04% confidence interval was applied which corresponds to a p-value less than 0.0496 for significance. CV death and HHF events were adjudicated by an independent clinical event committee and analysed based on the randomized set
***As pre-specified in the statistical testing procedure, a two-sided 99.9% confidence interval was applied which corresponds to a p-value less than 0.001 for significance. eGFR slope was analysed based on the treated set
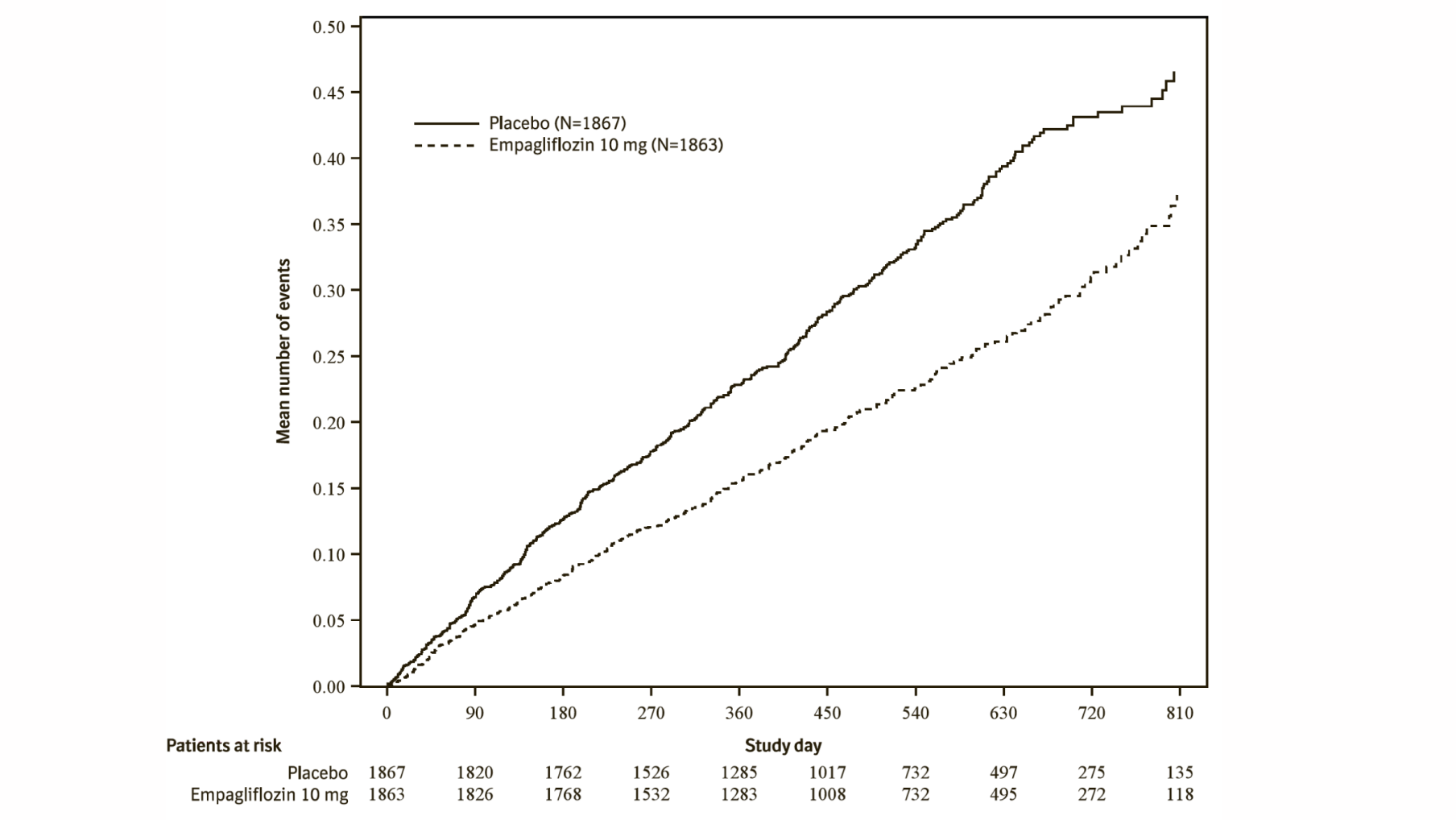
The results of the primary composite endpoint were generally consistent with a hazard ratio (HR) below 1 across the pre-specified subgroups, including heart failure patients with and without type 2 diabetes mellitus (see Figure 9).
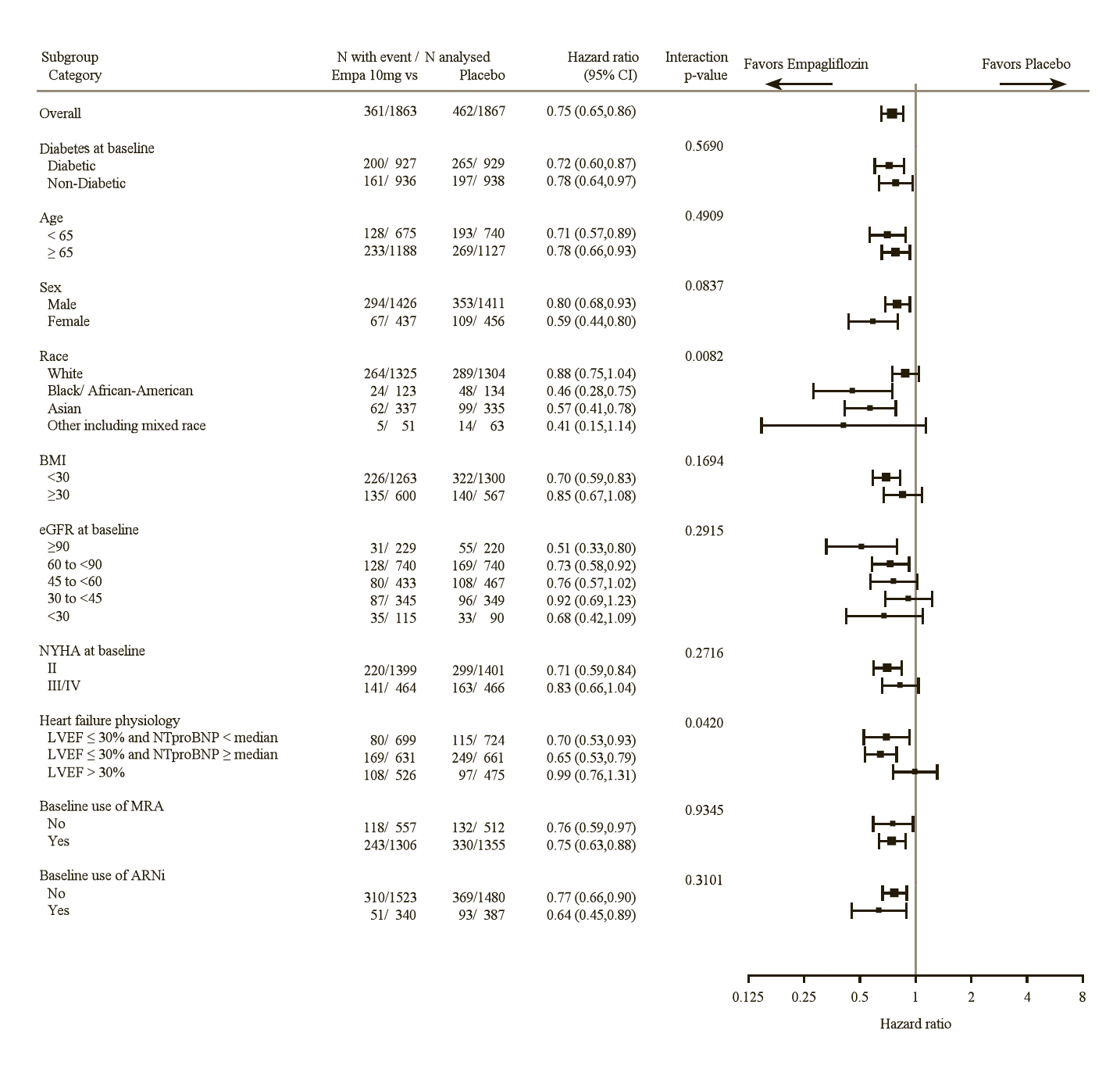
LVEF >30%: Includes both above and below the median NTproBNP. To be eligible for inclusion, patients with an LVEF >30% were required to meet a higher NTproBNP threshold than those with LVEF ≤30%, unless they additionally had a history of HHF within the past 12 months.
Renal Outcome
During treatment, eGFR decline over time was slower in the empagliflozin group compared to the placebo group (see Figure 10). Treatment with empagliflozin 10 mg significantly reduced the rate of eGFR decline and the effect was consistent across all pre-specified subgroups (see Table 20). Patients treated with empagliflozin experienced an initial drop in eGFR which returned towards baseline after treatment discontinuation supporting that haemodynamic changes play a role in the acute effects of empagliflozin on eGFR.
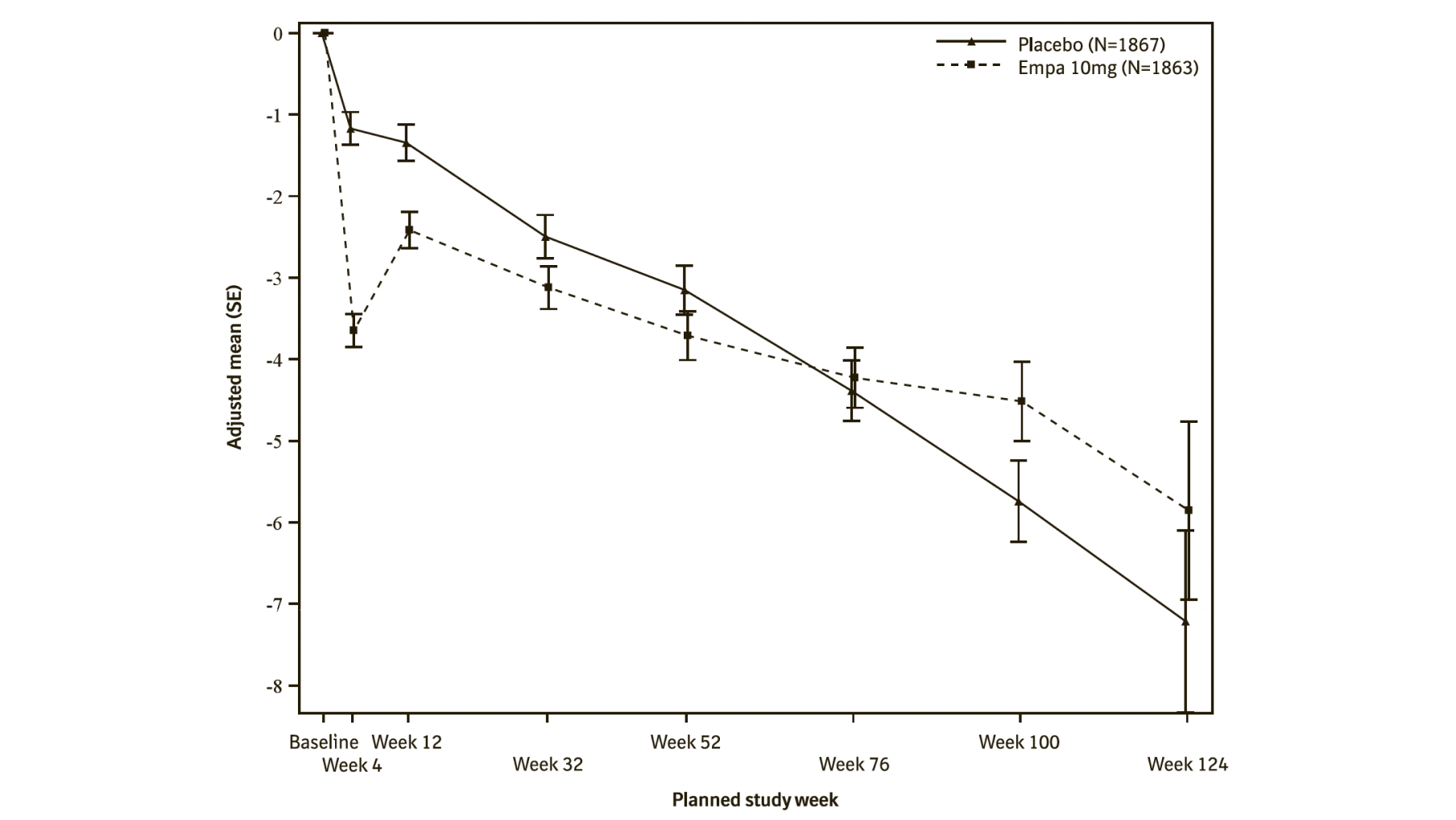
*eGFR (CKD-EPI) (mL/min/1.73 m²) MMRM results over time - randomized set. The number of patients who provided data at various time points (placebo, empagliflozin): at week 4 (1788, 1802); at week 12 (1729, 1756); at week 32 (1563, 1614); at week 52 (1211, 1228); at week 76 (801, 805); at week 100 (359, 386); and at week 124 (86, 91).
Empagliflozin (Jardiance) reduced the risk of the renal composite endpoint defined as time to first event of chronic dialysis or renal transplant or sustained reduction in eGFR compared with placebo (Table 21 and Figure 11).
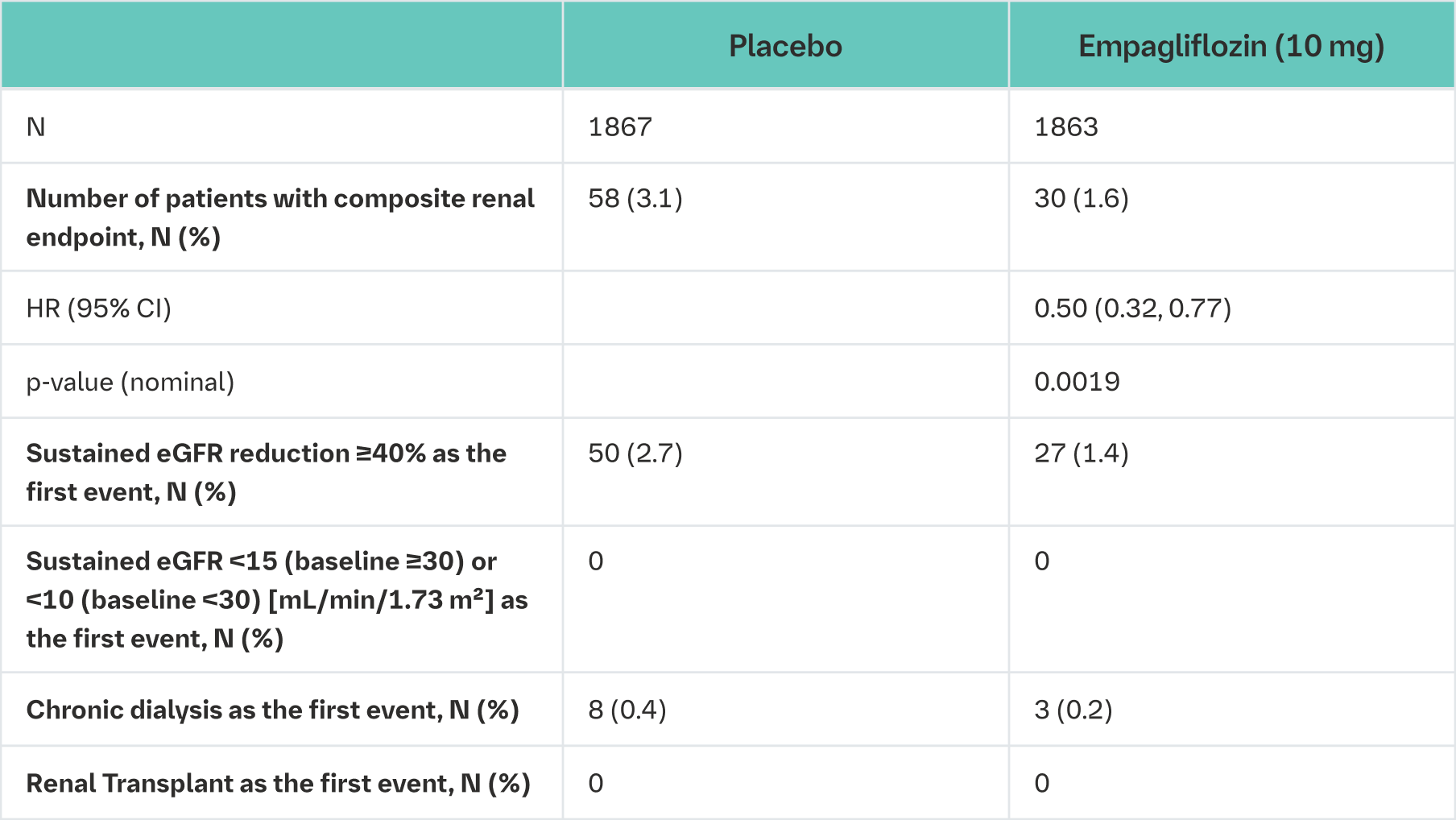
Composite renal endpoint is defined as chronic dialysis or renal transplant or sustained reduction of ≥40% eGFR (CKD−EPI)cr or sustained eGFR (CKD−EPI)cr <15 mL/min/1.73 m² (<10 mL/min/1.73 m² for patients with eGFR (CKD−EPI)cr <30 mL/min/1.73 m² at baseline). Dialysis is regarded as chronic if the frequency of dialysis is twice or more per week for at least 90 days.
An eGFR (CKD-EPI)cr reduction is considered sustained, if it is determined by two or more consecutive post baseline central laboratory measurements separated by at least 30 days (first to last of the consecutive eGFR values). If there is no additional measurement ≥30 days after the eGFR reduction is observed and the patient dies within 60 days of this measurement, then the eGFR reduction is also considered sustained.
The effect of empagliflozin on heart failure symptoms at week 52 was assessed as a patient reported outcome using the change from baseline in Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (CSS), which measures average of symptom frequency and burden for swelling, fatigue, and shortness of breath and physical limitations.
There was a greater improvement in the clinical summary score from baseline in the empagliflozin group than in the placebo group at Week 52 (placebo-corrected adjusted mean change from baseline 1.75, 95% CI 0.51 to 2.99, nominal p-value = 0.0058), driven by all domains included (symptom frequency, symptom burden, and physical limitations).
Empagliflozin in patients with heart failure and preserved ejection fraction
A randomised, double-blind, placebo-controlled study (EMPEROR-Preserved) was conducted in 5988 patients with chronic heart failure (NYHA II-IV) and preserved ejection fraction (LVEF >40%) to evaluate the efficacy and safety of empagliflozin 10 mg once daily as adjunct to standard of care therapy. The primary endpoint was the time to adjudicated first event of either cardiovascular (CV) death or hospitalisation for heart failure (HHF). Occurrence of adjudicated HHF (first and recurrent), and eGFR(CKD-EPI)cr slope of change from baseline were included in the confirmatory testing. Baseline therapy included ACE inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitor (80.7%), beta blockers (86.3%), mineralocorticoid receptor antagonists (37.5%) and diuretics (86.2%).
A total of 2997 patients were randomised to empagliflozin 10 mg (placebo: 2991) and followed for a median of 26.2 months. The study population consisted of 55.3% men and 44.7% women with a mean age of 71.9 years (range: 22-100 years), 43.0% were 75 years of age or older. 75.9% of the study population were White, 13.8% Asian and 4.3% Black/African American. At randomisation, 81.5% of patients were NYHA class II, 18.1% were class III and 0.3% were class IV. The EMPEROR-Preserved study population included patients with a LVEF <50% (33.1%), with a LVEF 50 to <60% (34.4%) and a LVEF ≥60% (32.5%). At baseline, the mean eGFR was 60.6 mL/min/1.73 m² and the median urinary albumin to creatinine ratio (UACR) was 21 mg/g. About half of the patients (50.1%) had an eGFR of ≥60 mL/min/1.73 m², 26.1% of 45 to <60 mL/min/1.73 m², 18.6% of 30 to <45 mL/min/1.73 m² and 4.9% 20 to <30 mL/min/1.73 m².
Empagliflozin was superior in reducing the risk of the primary composite endpoint of cardiovascular death or hospitalisation for heart failure compared with placebo. Additionally, empagliflozin significantly reduced the risk of occurrence of HHF (first and recurrent), and significantly reduced the rate of eGFR decline. (see Table 22).
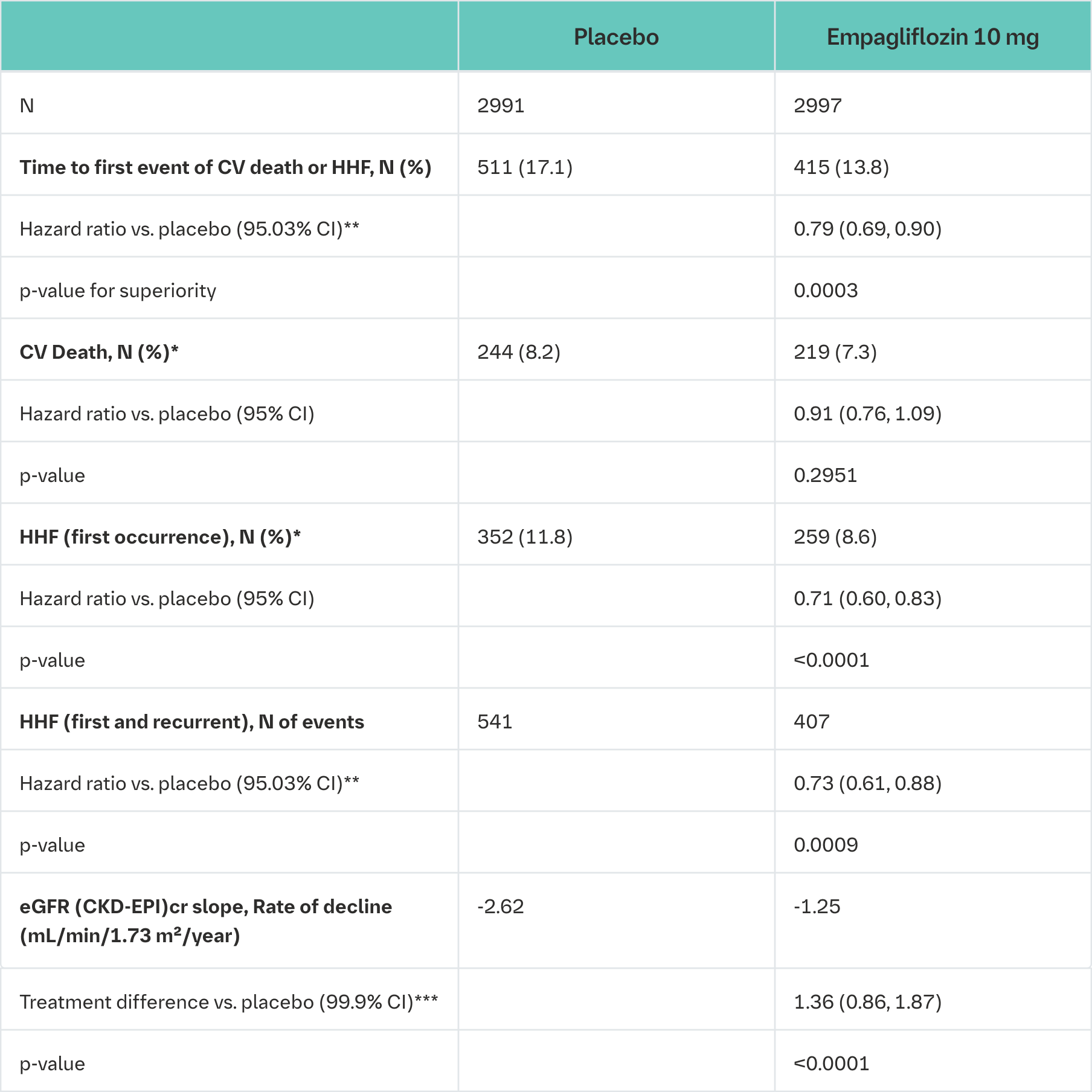
CV = cardiovascular, HHF = hospitalisation for heart failure, eGFR = Estimated glomerular filtration rate, CKD EPI = Chronic kidney disease epidemiology collaboration equation
*not controlled for type 1 error
**Due to an interim analysis, a two-sided 95.03% confidence interval was applied which corresponds to a p-value less than 0.0497 for significance. CV death and HHF events were adjudicated by an independent clinical event committee and analysed based on the randomised set
***As pre-specified in the statistical testing procedure, a two-sided 99.9% confidence interval was applied which corresponds to a p-value less than 0.001 for significance. eGFR slope was analysed based on the treated set
The results of the primary composite endpoint were consistent across each of the pre-specified subgroups categorized by e.g., LVEF, diabetes status or renal function down to an eGFR of 20 mL/min/1.73 m2 (see Figure 14).
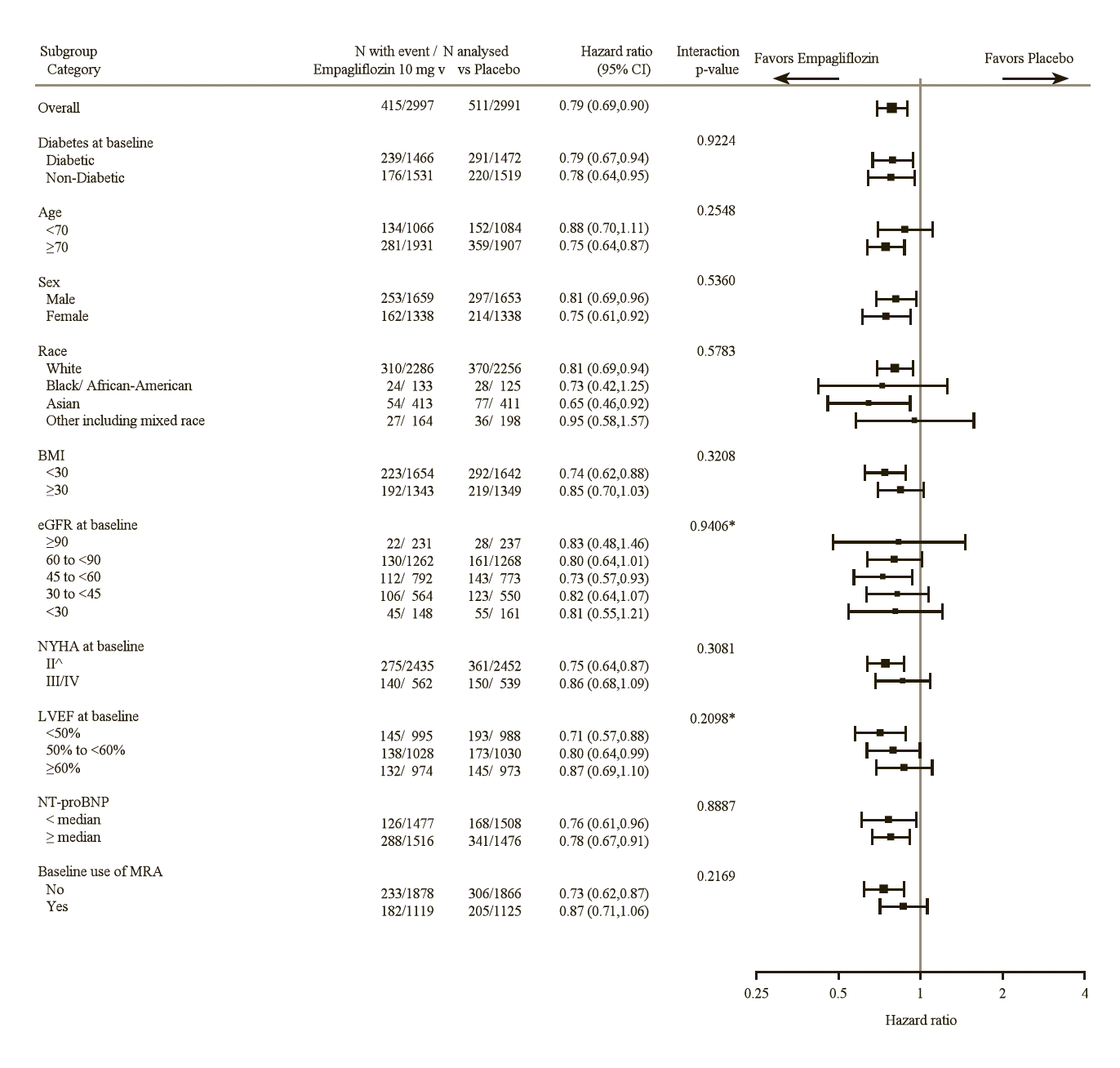
^4 patients with NYHA class I are counted in subgroup NYHA class II
*trend test
Renal outcome
During treatment, eGFR decline over time was slower in the empagliflozin group compared to the placebo group (see Figure 15). Treatment with empagliflozin 10 mg significantly reduced the rate of eGFR decline and the effect was consistent across all pre-specified subgroups (see Table 22). Patients treated with empagliflozin experienced an initial drop in eGFR which returned towards baseline after treatment discontinuation supporting that haemodynamic changes play a role in the acute effects of empagliflozin on eGFR.
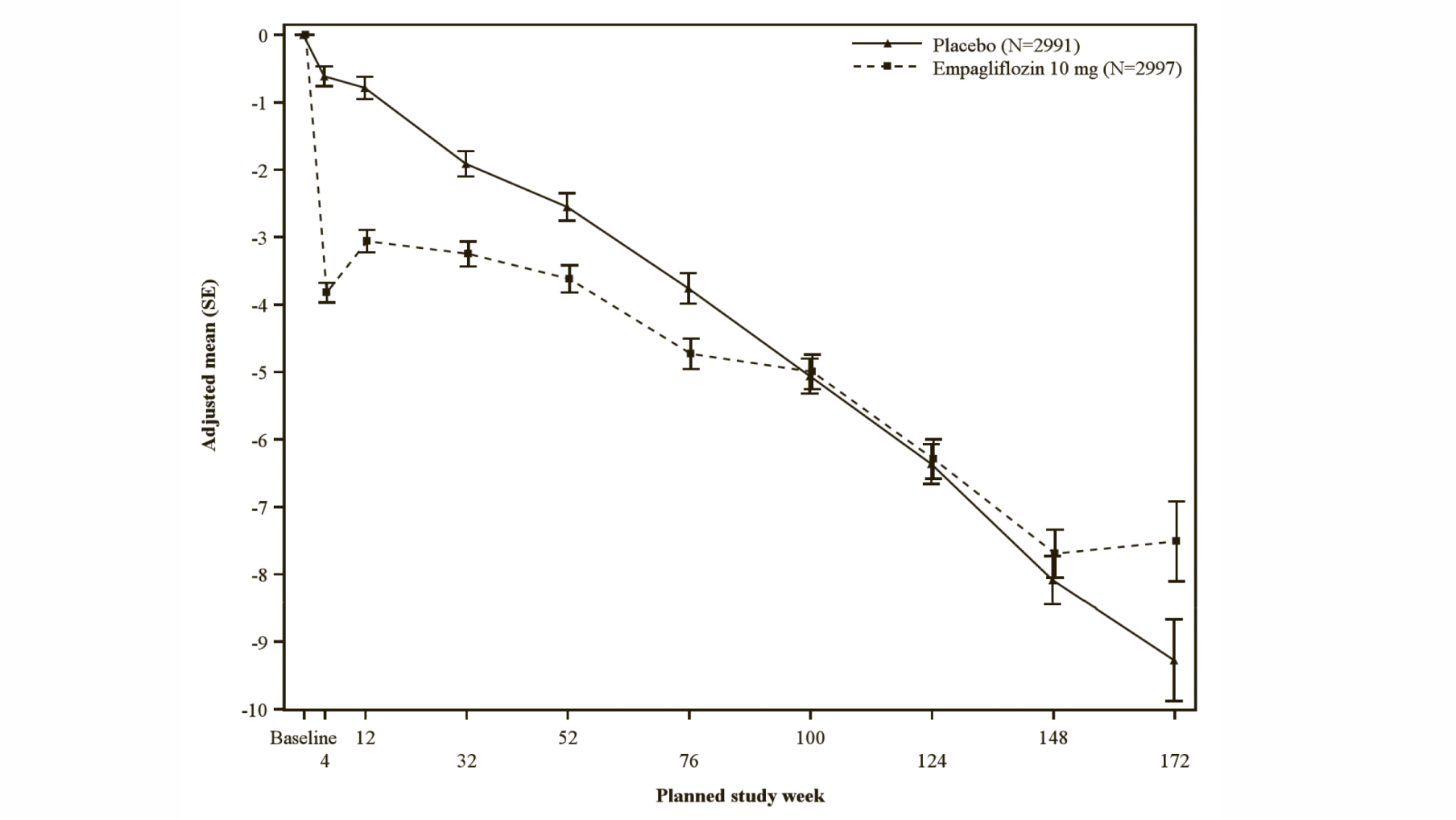
*eGFR (CKD-EPI) (mL/min/1.73 m2) MMRM results over time-randomised set. The number of patients who provided data at various time points (placebo, empagliflozin): at week 4 (2910, 2931); at week 12 (2820, 2854); at week 32 (2590, 2629); at week 52 (2457, 2474); at week 76 (2123, 2114); at week 100 (1548, 1550); at week 124 (1091, 1122), at week 148 (695, 686), at week 172 (231, 243) and at week 196 (16, 23).
In an analysis of the composite renal endpoint (defined as time to first event of chronic dialysis or renal transplant or sustained reduction in eGFR) the hazard ratio was 0.95 (95% CI 0.73 to 1.24, nominal p-value 0.7243).
The effect of empagliflozin on heart failure symptoms at week 52 was assessed as a patient-reported outcome using the change from baseline in Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (CSS), which measures average of symptom frequency and burden for swelling, fatigue, and shortness of breath and physical limitations.
There was a greater improvement in the clinical summary score from baseline in the empagliflozin group than in the placebo group at Week 52 (placebo-corrected adjusted mean change from baseline 1.32, 95% CI 0.45 to 2.19, nominal p-value = 0.0028), driven by the domains symptom frequency and symptom burden.
Empagliflozin in patients hospitalised for acute heart failure who have been stabilised
A randomised, double-blind, placebo-controlled study (EMPULSE) was conducted in 530 patients hospitalised for acute heart failure independent of LVEF (33.0% with de novo and 67.0% with decompensated chronic heart failure) who have been stabilised. The study evaluated the clinical benefit and safety of empagliflozin 10 mg once daily as adjunct to standard of care therapy. Treatment was initiated in the hospital and continued for 90 days. The primary endpoint was clinical benefit, a composite of death, number of heart failure events (including hospitalisations for heart failure, urgent heart failure visits and unplanned outpatient visits), time to first heart failure event and change from baseline in Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS) after 90 days of treatment assessed by the win ratio. Baseline therapy included angiotensin-converting-enzyme (ACE) inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitor (70.0%), beta blockers (79.4%) and diuretics (90.6%).
A total of 265 patients each were randomised to empagliflozin 10 mg or placebo and followed for a median of 98 days. The study population consisted of 66.2% men and 33.8% women with a mean age of 68.5 years (range: 22-98 years); 37.2% were 75 years of age or older. 77.9% of the study population were White, 10.8% Asian and 10.2% Black/African American. At randomisation, 2.6% of patients were in NYHA class I, 35.1% in class II, 52.6% in class III, 9.2% in class IV and 45.3% of patients had T2DM. The EMPULSE study population included 66.8% of patients with LVEF ≤40%, and 31.9% with LVEF >40%. At baseline, 36.6% of patients had an eGFR of ≥60 mL/min/1.73 m2, 22.8% of 45 to <60 mL/min/1.73 m2, 25.3% of 30 to <45 mL/min/1.73 m2, and 8.3% of 20 to <30 mL/min/1.73 m2.
In the primary analysis each patient in the empagliflozin group was compared to every patient in the placebo group within each stratum (de novo or decompensated chronic HF). Pairwise comparisons were performed in a hierarchical fashion using time to death followed by number of heart failure events, time to first heart failure event and a ≥5 point difference in change from baseline in KCCQ-TSS determining the burden and frequency of HF symptoms. The stratified win ratio was then calculated combining the number of wins in the Empagliflozin (Jardiance) group divided by the number of losses across strata.
Patients on empagliflozin were 36% more likely to experience a clinical benefit compared to placebo (win ratio 1.36, 95% CI 1.09, 1.68; p = 0.0054 (see Table 23)).
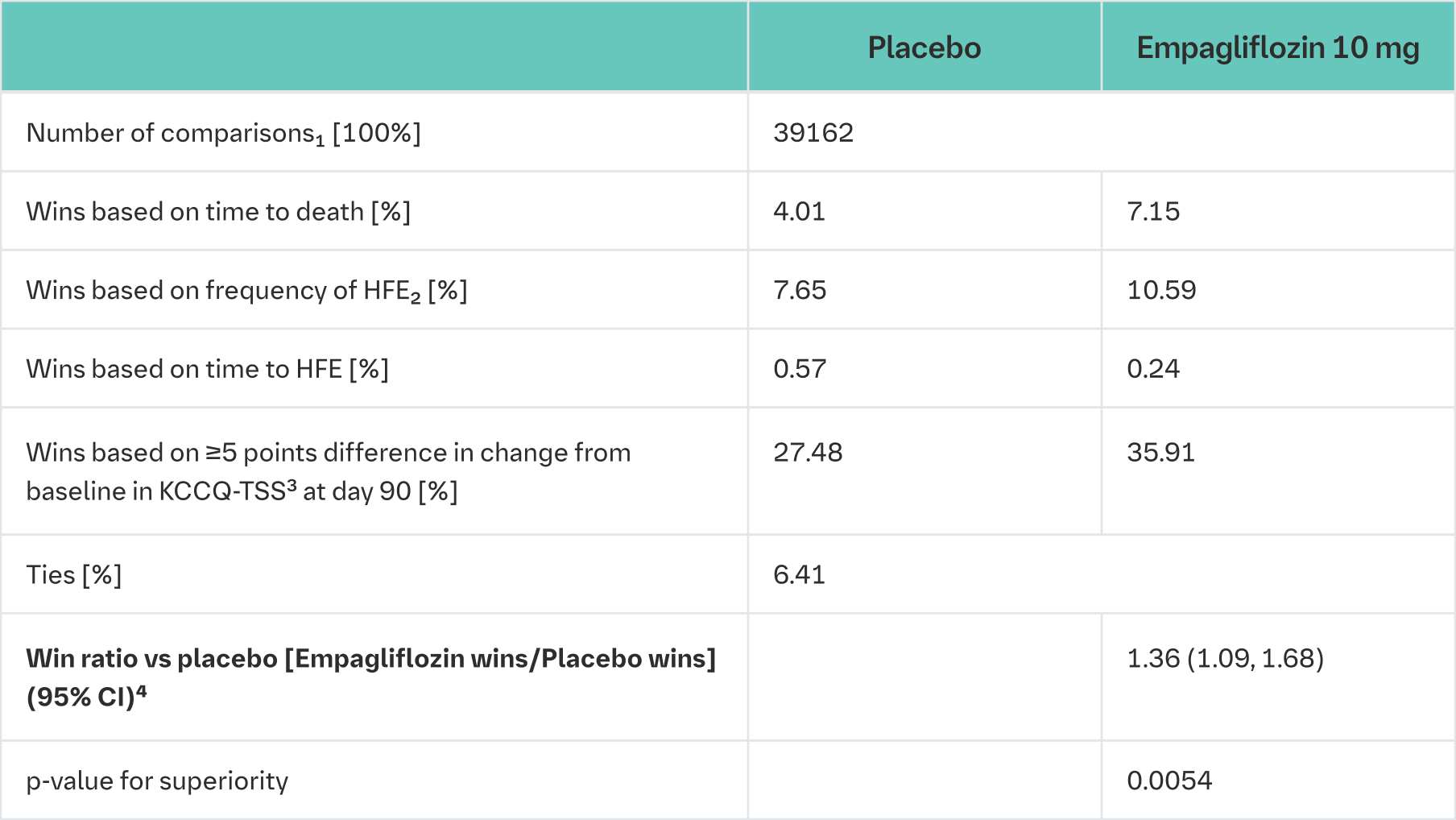
HFE = heart failure events, KCCQ-TSS = Kansas City Cardiomyopathy Questionnaire Total Symptom Score
1Pairs of patients were analysed within strata for a stratified win ratio, applying weights that are analogous to a Mantel−Haenszel approach
2Frequency based on events up to the earlier of the two censoring times
3Based on multiple imputation with 100 iterations
4Variance calculated using the asymptotic normal U statistics approach
The results of the primary endpoint were generally consistent across the pre-specified subgroups, including de novo heart failure and decompensated chronic heart failure, and were independent of LVEF.
Safety data from this study was in line with previous known safety profile of empagliflozin.
Chronic kidney disease
A randomised, double-blind, placebo-controlled study of empagliflozin 10 mg once daily (EMPA-KIDNEY) was conducted in 6609 patients with chronic kidney disease (eGFR ≥20 - <45 mL/min/1.73 m²; or eGFR ≥45 - <90 mL/min/1.73 m² with an urine albumin-to-creatinine ratio [UACR] ≥200 mg/g) to assess cardio-renal outcomes as adjunct to standard of care therapy. Treatment was allowed to be continued in patients receiving dialysis. The primary endpoint was the time to first occurrence of kidney disease progression (sustained ≥40% eGFR decline from randomisation, sustained eGFR <10 mL/min/1.73 m², end-stage kidney disease, or renal death) or CV death. All-cause hospitalisation (first and recurrent), first occurrence of hospitalisation for heart failure or CV death, and all-cause mortality were included in the confirmatory testing. Baseline therapy included an appropriate use of RAS-inhibitor (85.2% ACE inhibitor or angiotensin receptor blocker).
A total of 3304 patients were randomised to empagliflozin 10 mg (placebo: 3305) and followed for a median of 24.3 months. The study population consisted of 66.8% men and 33.2% women with a mean age of 63.3 years (range: 18-94 years), 23.0% were 75 years of age or older. 58.4% of the study population were White, 36.2% Asian and 4.0% Black/African American.
At baseline, the mean eGFR was 37.3 mL/min/1.73 m², 21.2% patients had an eGFR of ≥45 mL/min/1.73 m², 44.3% of 30 to <45 mL/min/1.73 m² and 34.5% <30 mL/min/1.73 m² including 254 patients with an eGFR <20 mL/min/1.73 m². The median UACR was 329 mg/g, 20.1% patients had an UACR <30 mg/g, 28.2% had an UACR 30 to ≤300 mg/g and 51.7% had an UACR >300 mg/g; 41.1% of patients had an UACR <200 mg/g. Primary causes of chronic kidney disease were diabetic nephropathy/diabetic kidney disease (31%), glomerular disease (25%), hypertensive/renovascular disease (22%) and other/unknown (22%).
Empagliflozin was superior in reducing the risk of the primary composite endpoint of kidney disease progression or CV death compared with placebo. In a pre-specified analysis, treatment with empagliflozin reduced the risk of end-stage kidney disease or CV death by 27% compared with placebo (HR 0.73, 95% CI 0.59 to 0.89, nominal p = 0.0023). Additionally, empagliflozin significantly reduced the risk of all-cause hospitalisation (first and recurrent). (see Table 24).
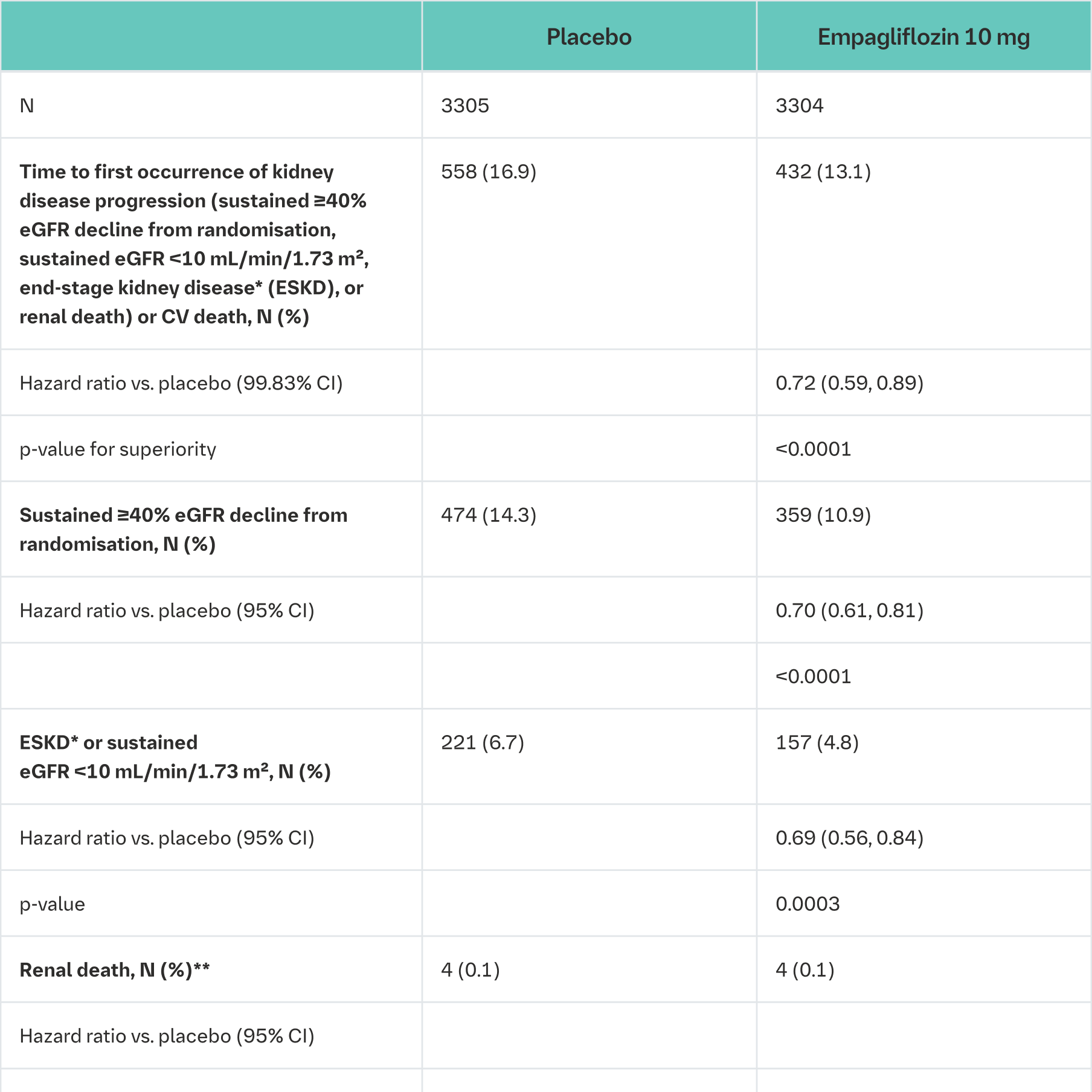
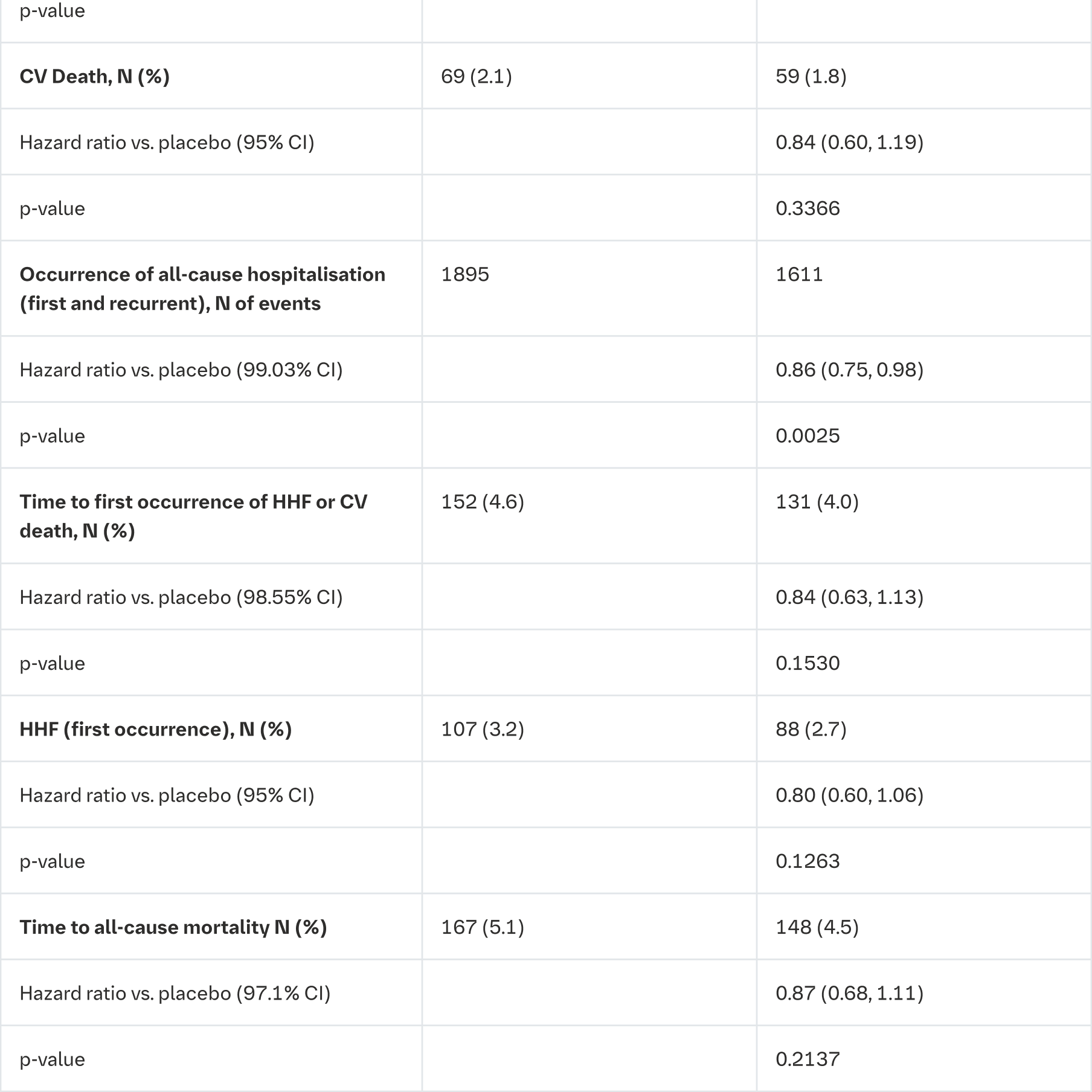
CV = cardiovascular, HHF = hospitalisation for heart failure, eGFR = estimated glomerular filtration rate
*End-stage kidney disease (ESKD) is defined as the initiation of maintenance dialysis or receipt of a kidney transplant
**There were too few events of renal death to compute a reliable hazard ratio
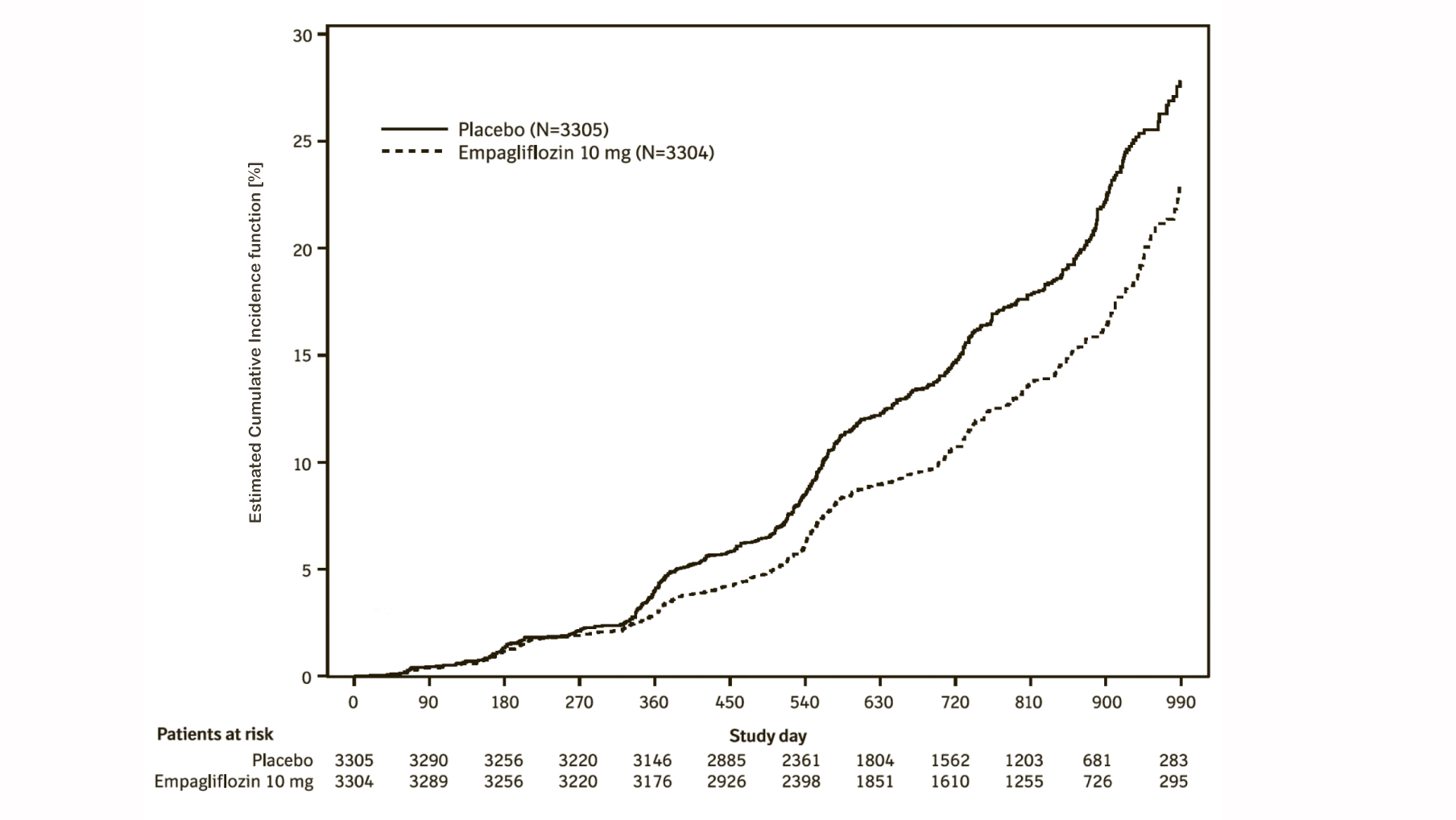
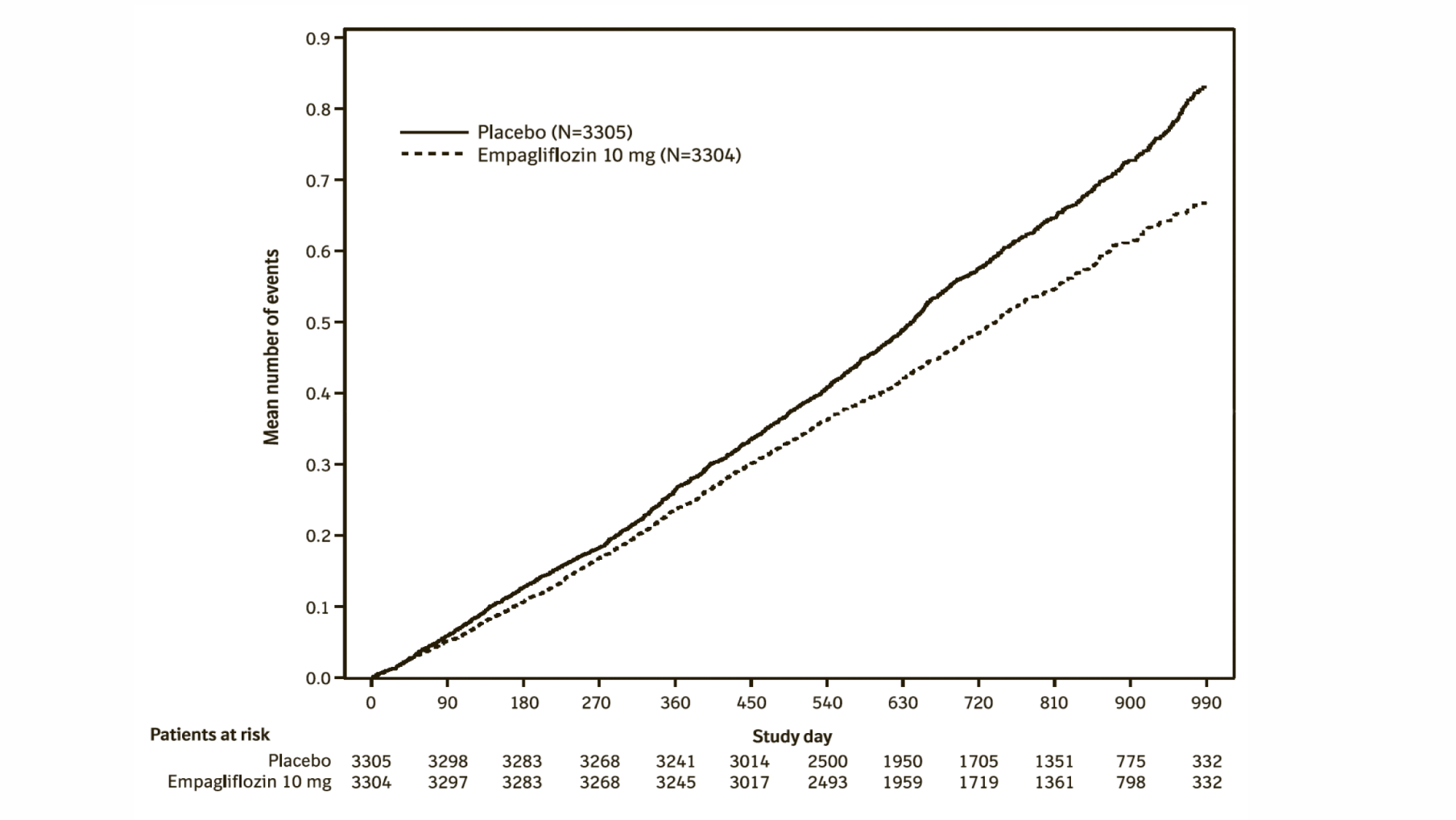
The results of the primary composite endpoint were generally consistent across the pre-specified subgroups, including eGFR categories, underlying cause of renal disease, diabetes status, or background use of RAS inhibitors. Treatment benefits were more clearly evident in patients with higher levels of albuminuria.
Empagliflozin slowed the annual rate of eGFR decline compared to placebo by 1.37 mL/min/1.73 m2/year (95% CI 1.16, 1.59), based on a pre-specified analysis of all eGFR measurements taken from the 2-month visit to the final follow-up visit. The observed effect was consistent irrespective of albuminuria, eGFR or diabetes status. These data further support the conclusion that Empagliflozin (Jardiance) is also likely to be effective in patients with less pronounced albuminuria.
12. Pharmacokinetics
Absorption
The pharmacokinetics of empagliflozin have been extensively characterized in healthy volunteers and patients with T2DM. After oral administration, empagliflozin was rapidly absorbed with peak plasma concentrations occurring at a median tmax 1.5 h post-dose. Thereafter, plasma concentrations declined in a biphasic manner with a rapid distribution phase and a relatively slow terminal phase. The steady state mean plasma AUC was 4740 nmol.h/L and Cmax was 687 nmol/L with 25 mg empagliflozin once daily. Systemic exposure of empagliflozin increased in a dose-proportional manner. The single-dose and steady-state pharmacokinetics parameters of empagliflozin were similar suggesting linear pharmacokinetics with respect to time. There were no clinically relevant differences in empagliflozin pharmacokinetics between healthy volunteers and patients with T2DM.
Administration of 25 mg empagliflozin after intake of a high-fat and high calorie meal resulted in slightly lower exposure; AUC decreased by approximately 16% and Cmax decreased by approximately 37%, compared to fasted condition. The observed effect of food on empagliflozin pharmacokinetics was not considered clinically relevant and empagliflozin may be administered with or without food.
Distribution
The apparent steady-state volume of distribution was estimated to be 73.8 L, based on a population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, the red blood cell partitioning was approximately 36.8% and plasma protein binding was 86.2%.
Biotransformation
No major metabolites of empagliflozin were detected in human plasma and the most abundant metabolites were three glucuronide conjugates (2-O-, 3-O-, and 6-O-glucuronide). Systemic exposure of each metabolite was less than 10% of total drug-related material. In vitro studies suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9.
Elimination
The apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 h and apparent oral clearance was 10.6 L/h based on the population pharmacokinetic analysis. The inter-subject and residual variabilities for empagliflozin oral clearance were 39.1% and 35.8%, respectively. With once-daily dosing, steady-state plasma concentrations of empagliflozin were reached by the fifth dose. Consistent with half-life, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state. Following administration of an oral [14C]-empagliflozin solution to healthy subjects, approximately 95.6% of the drug related radioactivity was eliminated in faeces (41.2%) or urine (54.4%). The majority of drug related radioactivity recovered in faeces was unchanged parent drug and approximately half of drug related radioactivity excreted in urine was unchanged parent drug.
Specific populations
Renal Impairment
In patients with mild (eGFR: 60 - <90 mL/min/1.73 m2), moderate (eGFR: 30 - <60 mL/min/1.73 m2), severe (eGFR: <30 mL/min/1.73 m2) renal impairment and patients with kidney failure/ESKD patients, AUC of empagliflozin increased by approximately 18%, 20%, 66%, and 48%, respectively, compared to subjects with normal renal function. Peak plasma levels of empagliflozin were similar in subjects with moderate renal impairment and kidney failure/ESKD compared to patients with normal renal function. Peak plasma levels of empagliflozin were roughly 20% higher in subjects with mild and severe renal impairment as compared to subjects with normal renal function. In line with the Phase I study, the population pharmacokinetic analysis showed that the apparent oral clearance of empagliflozin decreased with a decrease in eGFR leading to an increase in drug exposure. Based on pharmacokinetics, no dosage adjustment is recommended in patients with renal insufficiency.
Hepatic Impairment
In subjects with mild, moderate, and severe hepatic impairment according to the Child-Pugh classification, AUC of empagliflozin increased approximately by 23%, 47%, and 75% and Cmax by approximately 4%, 23%, and 48%, respectively, compared to subjects with normal hepatic function. Based on pharmacokinetics, no dosage adjustment is recommended in patients with hepatic impairment.
Body Mass Index (BMI)
No dosage adjustment is necessary based on BMI. Body mass index had no clinically relevant effect on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis.
Gender
No dosage adjustment is necessary based on gender. Gender had no clinically relevant effect on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis.
Race
No dosage adjustment is necessary based on race. Based on the population pharmacokinetic analysis, AUC was estimated to be 13.5% higher in Asian patients with a BMI of 25 kg/m2 compared to non-Asian patients with a BMI of 25 kg/m2.
Geriatric
Age did not have a clinically meaningful impact on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis.
Paediatric
Studies characterizing the pharmacokinetics of empagliflozin in paediatric patients have not been performed.
Toxicology
In general toxicity studies in rodents and dogs, signs of toxicity were observed at exposures greater than or equal to 10-times the clinical dose of 25 mg. Most toxicity was consistent with secondary pharmacology related to urinary glucose loss and included decreased body weight and body fat, increased food consumption, diarrhea, dehydration, decreased serum glucose and increases in other serum parameters reflective of increased protein metabolism, gluconeogenesis and electrolyte imbalances, urinary changes such as polyuria and glucosuria, and microscopic changes in kidney.
Carcinogenicity
Empagliflozin did not increase the incidence of tumours in female rats at doses up to the highest dose of 700 mg/kg/day, which corresponds to approximately 72- and 182-times the clinical AUC exposure associated with the 25 and 10 mg doses, respectively. In male rats, treatment-related benign vascular proliferative lesions (hemangiomas) of the mesenteric lymph node, were observed at 700 mg/kg/day, which corresponds to approximately 42- and 105-times the clinical exposure associated with the 25 mg and 10 mg doses, respectively. These tumours are common in rats and are unlikely to be relevant to humans. Empagliflozin did not increase the incidence of tumours in female mice at doses up to 1000 mg/kg/day, which corresponds to approximately 62- and 158-times the clinical exposure associated with the 25 mg and 10 mg doses, respectively. Empagliflozin induced renal tumours in male mice at 1000 mg/kg/day, which corresponds to approximately 45- and 113-times the clinical exposure associated with the 25 mg and 10 mg doses, respectively. The mode of action for these tumours is dependent on the natural predisposition of the male mouse to renal pathology and a metabolic pathway not reflective of humans. The male mouse renal tumours are considered not relevant to humans.
Genotoxicity
Empagliflozin is not genotoxic.
Reproduction Toxicity
Nonclinical studies show that empagliflozin crosses the placenta during late gestation to a very limited extent, but do not indicate direct or indirect harmful effects with respect to early embryonic development. Empagliflozin administered during the period of organogenesis was not teratogenic at doses up to 300 mg/kg in the rat or rabbit, which corresponds to approximately 48- and 122-times or 128- and 325-times the clinical dose of empagliflozin based on AUC exposure associated with the 25 mg and 10 mg doses, respectively. Doses of empagliflozin causing maternal toxicity in the rat also caused the malformation of bent limb bones at exposures approximately 155- and 393-times the clinical dose associated with the 25 mg and 10 mg doses, respectively. Maternally toxic doses in the rabbit also caused increased embryofetal loss at doses approximately 139- and 353-times the clinical dose associated with the 25 mg and 10 mg doses, respectively.
In pre- and postnatal toxicity studies in rats, reduced weight gain in offspring was observed at maternal exposures approximately 4- and 11-times the clinical dose associated with the 25 mg and 10 mg doses, respectively.
In a juvenile toxicity study in the rat, when empagliflozin was administered from postnatal day 21 until postnatal day 90, non-adverse, minimal to mild renal tubular and pelvic dilation in juvenile rats was seen only at 100 mg/kg/day, which approximates 11-times the maximum clinical dose of 25 mg. These findings were absent after a 13 weeks drug-free recovery period.
Availability
Tablets of
10 mg Alu/PVC blister pack of 10’s (Box of 30’s)
25 mg Alu/PVC blister pack of 10’s (Box of 30’s)
Caution: Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription.
For any suspected adverse drug reaction, please seek medical attention immediately. Report this to the FDA: www.fda.gov.ph and email us at PV_local_Philippines@boehringer-ingelheim.com.
Store at temperatures not exceeding 30°C.
Manufactured by
Rottendorf Pharma GmbH
Ostenfelder Str. 51-61
59320 Ennigerloh, Germany
Packed by
Rottendorf Pharma GmbH
Am Fleigendahl 3
59320 Ennigerloh, Germany
Imported by
Boehringer Ingelheim (Philippines), Inc.
23rd Floor BDO Towers Valero Bldg.
8741 Paseo de Roxas, Bel-Air
Makati City, Metro Manila
DR-XY48713 (10 mg Tablet)
DR-XY48712 (25 mg Tablet)
Date of First Authorization: 02 May 2023
Date of Revision of Text: 14 November 2022
